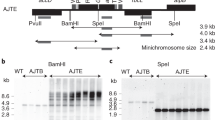
Abstract
Transgenic plants have become attractive systems for production of human therapeutic proteins because of the reduced risk of mammalian viral contaminants, the ability to do large scale-up at low cost, and the low maintenance requirements. Here we report a feasibility study for production of a human therapeutic protein through transplastomic transformation technology, which has the additional advantage of increased biological containment by apparent elimination of the transmission of transgenes through pollen. We show that chloroplasts can express a secretory protein, human somatotropin, in a soluble, biologically active, disulfide-bonded form. High concentrations of recombinant protein accumulation are observed (>7% total soluble protein), more than 300-fold higher than a similar gene expressed using a nuclear transgenic approach. The plastid-expressed somatotropin is nearly devoid of complex post-translational modifications, effectively increasing the amount of usable recombinant protein. We also describe approaches to obtain a somatotropin with a non-methionine N terminus, similar to the native human protein. The results indicate that chloroplasts are a highly efficient vehicle for the potential production of pharmaceutical proteins in plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goddijn, O.J.M. & Pen, J. Plants as bioreactors. TIBTECH 13, 379–387 (1995).
Mason, H.S. & Arntzen, C.J. Transgenic plants as vaccine production systems. TIBTECH 13, 388– 392 (1995).
Cramer, C.L. et al. Bioproduction of human enzymes in transgenic tobacco. Ann. NY Acad. Sci. 792, 63–72 (1996).
Ma, J.K.-C. & Hein, M.B. Antibody production and engineering in plants. Ann. NY Acad. Sci. 792, 73– 81 (1996).
Zeitlin, L. et al. A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nat. Biotechnol. 16, 1361–1364 (1998).
McCormick, A.A. et al. Rapid production of specific vaccines for lymphoma by expression of the tumor-derived single-chain Fv epitopes in tobacco plants. Proc. Natl. Acad. Sci. USA 96, 703– 708 (1999).
Tacket, C.O. et al. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med. 4, 607–609 (1998).
Svab, Z., Hajdukiewicz, P.T.J. & Maliga, P. Stable transformation of plastids in higher plants. Proc. Natl. Acad. Sci. USA 87, 8526– 8530 (1990).
Svab, Z. & Maliga, P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc. Natl. Acad. Sci. USA 90, 913–917 (1993).
Sikdar, S.R., Serino, G., Chaudhuri, S. & Maliga, P. Plastid transformation in Arabidopsis thaliana. Plant Cell Reports 18, 20–24 ( 1998).
Sidorov, V.A. et al. Stable chloroplast transformation in potato, use of green fluorescent protein as a plastid marker. Plant J. 19 , 209–216 (1999).
Maliga, P. Towards plastid transformation in flowering plants. TIBTECH 11, 101–106 (1993).
Scott, S.E. & Wilkinson, M.J. Low probability of chloroplast movement from oilseed rape (Brassica napus) into wild Brassica rapa . Nat. Biotechnol. 17, 390– 392 (1999).
Daniell, H., Datta, R., Varma, S., Gray, S. & Lee, S.-B. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat. Biotechnol. 16, 345–348 (1998).
Ruelland, E. & Miginiac-Maslow, M. Regulation of chloroplast enzyme activities by thioredoxins: activation or relief from inhibition? Trends Plant Sci. 4, 136–141 (1999).
Tritos, N.A. & Mantzoros, C.S. Recombinant growth hormone: old and novel uses. Am. J. Med. 105, 44– 57 (1998).
Chawla, R.K., Parks, J.S. & Rudman, D. Structural variants of human growth hormone: biochemical, genetic and clinical aspects. Ann. Rev. Med. 34, 519–547 (1983).
Cronin, M.J. Pioneering recombinant growth hormone manufacturing: pounds produced per mile of height. J. Pediatr. 131, S5– S7 (1997).
Goeddel, D.V. et al. Direct expression in Escherichia coli of a DNA sequence coding for human growth hormone. Nature 281, 544–548 (1979).
Gray, G.L., McKeown, K.A., Jones, A.J.S., Seeburg, P.H. & Heyneker, H.L., Pseudomonas aeruginosa secretes and correctly processes human growth hormone. Bio/Technology 2, 161–165 ( 1984).
Becker, G.W. & Hsiung, H.M. Expression, secretion and folding of human growth hormone in Escherichia coli. FEBS Lett. 204, 145–150 ( 1986).
Baker, R.T. Protein expression using ubiquitin fusion and cleavage. Curr. Opin. Biotechnol. 7, 541–546 ( 1996).
Vierstra, R.D. Proteolysis in plants: mechanisms and functions. Plant Mol. Biol. 32, 275–302 ( 1996).
Staub, J.M. & Maliga, P. Translation of psbA mRNA is regulated by light via the 5′-untranslated region in tobacco plastids. Plant J. 6, 547–553 ( 1994).
Zoubenko, O.V., Allison, L.A., Svab, Z. & Maliga, P., Efficient targeting of foreign genes into the tobacco plastid genome. Nucleic Acids Res. 22, 3819–3824 ( 1994).
Lei, J., Chen, D.A. & Regnier, F.E. Rapid verification of disulfide linkages in recombinant human growth hormone by tandem column tryptic mapping. J. Chromatogr. 808, 121–131 ( 1998).
Kim, J. & Mayfield, S.P. Protein disulfide isomerase as a regulator of chloroplast translational activation. Science 278, 1954–1957 (1997).
Moore, J.A. et al. Equivalent potency and pharmokinetics of recombinant human growth hormones with or without an N-terminal methionine. Endocrinology 122, 2920–2926 ( 1988).
Meinnel, T., Mechulam, Y. & Blanquet, S. Methionine as translation start signal: a review of the enzymes of the pathway in Escherichia coli. Biochimie 75, 1061–1075 ( 1993).
McBride, K.E. et al. Amplification of a chimeric Bacillus gene in chloroplasts leads to an extraordinary level of an insecticidal protein in tobacco. Bio/Technology 13, 362–365 (1995).
Guda, C., Lee, S.-B. & Daniell, H. Stable expression of a biodegradable protein-based polymer in tobacco chloroplasts. Plant Cell Reports 19, 257–262 (2000).
Smyth, D.R. Gene silencing: cosuppression at a distance. Curr. Biol. 7, R793–795 (1997).
Bosch, D., Smal, J. & Krebbers, E. A trout growth hormone is expressed, correctly folded and partially glycosylated in the leaves but not the seeds of transgenic plants. Transgenic Res. 3, 304– 310 (1994).
Studier, F.W., Rosenberg, A.H., Dunn, J.J. & Dubendorff, J.W. Use of T7 RNA Polymerase to direct expression of cloned genes. Methods Enzymol. 185, 60–89 (1990).
Dattani, M.T. et al. G120R, a human growth hormone antagonist, shows zinc-dependent agonist and antagonist activity on Nb2 cells. J. Biol. Chem. 270, 9222–9226 (1995).
Acknowledgements
We thank Robin Weinberg for a ubiquitin–hST fusion plasmid, Titik Dian for assistance with protein purification, Dawn Dufield for mass spectrometry analysis, Skooter Jennings for amino acid analysis, and Pal Maliga, Rutgers University, for the pPRV plasmids. We also thank Michael Montague for a critical reading of the manuscript and Ganesh Kishore, Michael Montague, Julio Baez, Doug Taylor, and Stephen Padgette for support throughout this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Staub, J., Garcia, B., Graves, J. et al. High-yield production of a human therapeutic protein in tobacco chloroplasts . Nat Biotechnol 18, 333–338 (2000). https://doi.org/10.1038/73796
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/73796
This article is cited by
-
The lyophilized chloroplasts store synthetic DARPin G3 as bioactive encapsulated organelles
Journal of Biological Engineering (2023)
-
Chloroplast transformation for bioencapsulation and oral delivery using the immunoglobulin G fragment crystallizable (Fc) domain
Scientific Reports (2023)
-
The production of the first functional antibody mimetic in higher plants: the chloroplast makes the DARPin G3 for HER2 imaging in oncology
Biological Research (2022)
-
Plant Platforms for Efficient Heterologous Protein Production
Biotechnology and Bioprocess Engineering (2021)