Abstract
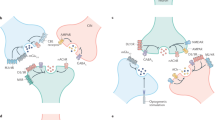
Fast excitatory synaptic transmission through vertebrate autonomic ganglia is mediated by postsynaptic nicotinic acetylcholine receptors (nAChRs). We demonstrate a unique postsynaptic receptor microheterogeneity on chick parasympathetic ciliary ganglion neurons—under one presynaptic terminal, nAChRs and glycine receptors formed separate but proximal clusters. Terminals were loaded with [3H]glycine via the glycine transporter-1 (GlyT-1), which localized to the cholinergic presynaptic terminal membrane; depolarization evoked [3H]glycine release that was calcium independent and blocked by the GlyT-1 inhibitor sarcosine. Ganglionic synaptic transmission mediated by nAChRs was attenuated by glycine. Coexistence of separate clusters of receptors with opposing functions under one terminal contradicts Dale's principle and provides a new mechanism for modulating synaptic activity in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhang, Z. W. & Berg, D.K. Patch-clamp analysis of glycine-induced currents in chick ciliary ganglion neurons. J. Physiol. (Lond.) 487, 395–405 ( 1995).
Landmesser, L. & Pilar, G. The onset and development of transmission in the chick ciliary ganglion. J. Physiol. (Lond.) 222, 691–713 (1972).
Landmesser, L. & Pilar, G. Synaptic transmission and cell death during normal ganglionic development. J. Physiol. (Lond.) 241, 737–749 (1974).
Martin, A. R. & Pilar, G. Dual mode of synaptic transmission in the avian ciliary ganglion. J. Physiol. (Lond.) 168, 443–463 (1963).
Martin, A. R. & Pilar, G. Transmission through the ciliary ganglion of the chick. J. Physiol. (Lond.) 168, 464 –475 (1963).
Reiner, A. et al. Neurotransmitter organization of the nucleus of Edinger-Westphal and its projection to the avian ciliary ganglion. Vis. Neurosci. 6, 451–472 ( 1991).
Engisch, K. L. & Fischbach, G.D. The development of ACh- and GABA-activated currents in embryonic chick ciliary ganglion neurons in the absence of innervation in vivo. J. Neurosci. 12, 1115–1125 (1992).
Arenella, L. S., Oliva, J. M. & Jacob, M.H. Reduced levels of acetylcholine receptor expression in chick ciliary ganglion neurons developing in the absence of innervation . J. Neurosci. 13, 4525– 4537 (1993).
Dale, H. Pharmacology and nerve-endings. Proc. R. Soc. Med. (Lond.) 28, 319–332 (1935).
Nicoll, R. A. & Malenka, R.C. A tale of two transmitters. Science 281, 360–361 ( 1998).
Jacob, M. H., Lindstrom, J. M. & Berg, D.K. Surface and intracellular distribution of a putative neuronal nicotinic acetylcholine receptor. J. Cell Biol. 103, 205–214 (1986).
Jacob, M.H. Acetylcholine receptor expression in developing chick ciliary ganglion neurons . J. Neurosci. 11, 1701– 1712 (1991).
Williams, B. M. et al. The long internal loop of the alpha 3 subunit targets nAChRs to subdomains within individual synapses on neurons in vivo. Nat. Neurosci. 1, 557–562 (1998).
Jacob, M. H. & Berg, D.K. The ultrastructural localization of α-bungarotoxin binding sites in relation to synapses on chick ciliary ganglion neurons. J. Neurosci. 3, 260– 271 (1983).
Jacob, M. H., Berg, D. K. & Lindstrom, J.M. Shared antigenic determinant between the Electrophorus acetylcholine receptor and a synaptic component on chick ciliary ganglion neurons. Proc. Natl. Acad. Sci. USA 81, 3223–3227 (1984).
Vernallis, A. B., Conroy, W. G. & Berg, D.K. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor sites. Neuron 10, 451–464 (1993).
Schroder, S., Hoch, W., Becker, C. M., Grenningloh, G. & Betz, H. Mapping of antigenic epitopes on the alpha 1 subunit of the inhibitory glycine receptor. Biochemistry 30 , 42–47 (1991).
Kirsch, J., Wolters, I., Triller, A. & Betz, H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature 366, 745–748 ( 1993).
Colin, I., Rostaing, P., Augustin, A. & Triller, A. Localization of components of glycinergic synapses during rat spinal cord development. J. Comp. Neurol. 398, 359– 372 (1998).
Feng, G. et al. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282, 1321–1324 (1998).
Prior, P. et al. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron 8, 1161–1170 (1992).
Pfeiffer, F., Simler, R., Grenningloh, G. & Betz, H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc. Natl. Acad. Sci. USA 81, 7224–7227 (1984).
Meyer, G., Kirsch, J., Betz, H. & Langosch, D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron 15, 563–572 ( 1995).
Kins, S., Kuhse, J., Laube, B., Betz, H. & Kirsch, J. Incorporation of a gephyrin-binding motif targets NMDA receptors to gephyrin-rich domains in HEK 293 cells. Eur. J. Neurosci. 11, 740–744 ( 1999).
Zhang, Z. W., Vijayaraghavan, S. & Berg, D.K. Neuronal acetylcholine receptors that bind alpha-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron 12, 167–177 ( 1994).
Marwitt, R., Pilar, G. & Weakly, J.N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 25, 317– 334 (1971).
McEachern, A. E., Margiotta, J. F. & Berg, D.K. Gamma-Aminobutyric acid receptors on chick ciliary ganglion neurons in vivo and in cell culture. J. Neurosci. 5, 2690–2695 ( 1985).
Sorimachi, M., Rhee, J. S., Shimura, M. & Akaike, N. Mechanisms of GABA- and glycine-induced increases of cytosolic Ca2+ concentrations in chick embryo ciliary ganglion cells. J. Neurochem. 69, 797–805 (1997).
McGale, E. H., Pye, I. F., Stonier, C., Hutchinson, E. C. & Aber, G.M. Studies of the inter-relationship between cerebrospinal fluid and plasma amino acid concentrations in normal individuals. J. Neurochem. 29, 291–297 (1977).
Attwell, D., Barbour, B. & Szatkowski, M. Nonvesicular release of neurotransmitter. Neuron 11, 401–407 ( 1993).
Zafra, A. F. et al. Glycine transporters are differentially expressed among CNS cells J. Neurosci. 15, 3952– 3969 (1995).
Liu, W., Leibach, F. H. & Ganapathy, V. Characterization of the glycine transport system GLYT 1 in human placental choriocarcinoma cells (JAR). Biochim. Biophys. Acta 1194, 176–184 ( 1994).
Pow, D.V. Transport is the primary determinant of glycine content in retinal neurons J. Neurochem. 70, 2628– 2636 (1998).
Schwartz, E.A. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science 238, 350– 355 (1987).
Coggan, J. S., Paysan, J., Conroy, W. G. & Berg, D.K. Direct recording of nicotinic responses in presynaptic nerve terminals. J. Neurosci. 17, 5798–5806 (1997).
Dumoulin, A. et al. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J. Cell Sci. 112, 811–823 ( 1999).
O'Malley, D. M., Sandell, J. H. & Masland, R.H. Co-release of acetylcholine and GABA by the starburst amacrine cells. J. Neurosci. 12, 1394– 1408 (1992).
Jo, Y. H. & Schlichter, R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat. Neurosci. 2, 241–245 (1999).
Smith, M. A., Stollberg, J., Lindstrom, J. M. & Berg, D.K. Characterization of a component in chick ciliary ganglia that cross-reacts with monoclonal antibodies to muscle and electric organ acetylcholine receptor . J. Neurosci. 5, 2726– 2731 (1985).
Horch, H. L. & Sargent, P.B. Perisynaptic surface distribution of multiple classes of nicotinic acetylcholine receptors on neurons in the chicken ciliary ganglion. J. Neurosci. 15, 7778–7795 (1995).
Horch, H. L. & Sargent, P.B. Effects of denervation on acetylcholine receptor clusters on frog cardiac ganglion neurons as revealed by quantitative laser scanning confocal microscopy. J. Neurosci. 16 , 1720–1729 (1996).
Wang, B. L. & Larsson, L.I. Simultaneous demonstration of multiple antigens by indirect immunofluorescence or immunogold staining. Novel light and electron microscopical double and triple staining method employing primary antibodies from the same species. Histochemistry 83, 47–56 (1985).
Buckley, K. & Kelly, R.B. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells . J. Cell Biol. 100, 1284– 1294 (1985).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: a Laboratory Manual (Cold Spring Harbor Press, New York, 1989).
Ikonomov, O. C., Kulesa, M. C., Shisheva, A. C. & Jacob, M.H. Innervation and target tissue interactions induce Rab-GDP dissociation inhibitor (GDI) expression during peripheral synapse formation in developing chick ciliary ganglion neurons in situ. J. Neurosci. 18, 6331–6339 (1998).
Bartel, P., Chien, C. T., Sternglanz, R. & Fields, S. Elimination of false positives that arise in using the two-hybrid system. Biotechniques 14, 920–924 (1993).
Hollenberg, S. M., Sternglanz, R., Cheng, P. F. & Weintraub, H. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol. Cell Biol. 15, 3813–3822 (1995).
Jacob, M. H. & Berg, D.K. The distribution of acetylcholine receptors in chick ciliary ganglion neurons following disruption of ganglionic connections. J. Neurosci. 8, 3838– 3849 (1988).
Boyd, R. T., Jacob, M. H., McEachern, A. E., Caron, S. & Berg, D.K. Nicotinic acetylcholine receptor mRNA in dorsal root ganglion neurons. J. Neurobiol. 22, 1–14 (1991).
Ryan, T. A. et al. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron 11, 713– 724 (1993).
Acknowledgements
We acknowledge Heinrich Betz for providing glycine receptor and gephyrin antibodies and clones, Yimen Ge (Massachusetts General Hospital, Harvard Medical School) for assistance with the laser-scanning confocal microscope and Kathleen Dunlap, Daniel Jay and Tim Turner for advice and comments on the manuscript. This work was supported by NIH grant 21725 to M.H.J.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsen, G., Williams, B., Allaire, P. et al. Receptors with opposing functions are in postsynaptic microdomains under one presynaptic terminal. Nat Neurosci 3, 126–132 (2000). https://doi.org/10.1038/72066
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72066
This article is cited by
-
B-Glycine as a marker for β cell imaging and β cell mass evaluation
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Segregation of glutamatergic and cholinergic transmission at the mixed motoneuron Renshaw cell synapse
Scientific Reports (2017)
-
Role of Glycine Receptors in Glycine-Induced LTD in Hippocampal CA1 Pyramidal Neurons
Neuropsychopharmacology (2011)
-
The Mechanism of Allosteric Interaction of Cytoplasmic and Extracellular Cl- in the Glial Glycine Transporter (hGlyTlb)
Doklady Biological Sciences (2005)
-
Molecular heterogeneity of central synapses: afferent and target regulation
Nature Neuroscience (2001)