Abstract
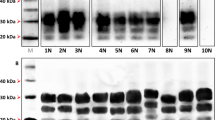
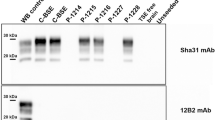
More than a million cattle infected with bovine spongiform encephalopathy (BSE) may have entered the human food chain1. Fears that BSE might transmit to man were raised when atypical cases of Creutzfeldt–Jakob disease (CJD), a human transmissible spongiform encephalopathy (TSE), emerged in the UK2,3. In BSE and other TSE diseases, the conversion of the protease-sensitive host prion protein (PrP-sen) to a protease-resistant isoform (PrP-res) is an important event in pathogenesis4,5,6,7. Biological aspects of TSE diseases are reflected in the specificities of in vitro PrP conversion reactions8,9,10,11,12. Here we show that there is a correlation between in vitro conversion efficiencies and known transmissibilities of BSE, sheep scrapie and CJD. On this basis, we used an in vitro system to gauge the potential transmissibility of scrapie and BSE to humans. We found limited conversion of human PrP-sen to PrP-res driven by PrP-res associated with both scrapie (PrPSc) and BSE (PrPBSE). The efficiencies of these heterologous conversion reactions were similar but much lower than those of relevant homologous conversions. Thus the inherent ability of these infectious agents of BSE and scrapie to affect humans following equivalent exposure may be finite but similarly low.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Anderson, R. M. et al. Transmission dynamics and epidemiology of BSE in British cattle. Nature 382, 779–788 (1996).
Will, R. G. et al. Anew variant of Creutzfeldt–Jakob disease in the UK. Lancet 347, 921–925 (1996).
Collinge, J., Sidle, K. C. L., Meads, J., Ironside, J. & Hill, A. F. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383, 685–690 (1996).
Hope, J. et al. Fibrils from brains of cows with new cattle disease contain scrapie-associated protein. Nature 336, 390–392 (1988).
Bolton, D. C., McKinley, M. P. & Prusiner, S. B. Identification of a protein that purifies with the scrapie prion. Science 218, 1309–1311 (1982).
Weissmann, C. Molecular biology of transmissible spongiform encephalopathies. FEBS Lett. 389, 3–11 (1996).
Caughey, B. & Chesebro, B. Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biol. 7, 56–62 (1997).
Kocisko, D. A. et al. Cell-free formation of protease-resistant prion protein. Nature 370, 471–474 (1994).
Kocisko, D. A. et al. Species specificity in the cell-free conversion of prion protein to protease-resistant forms: a model for the scrapie species barrier. Proc. Natl Acad. Sci. USA 92, 3923–3927 (1995).
Bessen, R. A. et al. Non-genetic propagation of strain-specific phenotypes of scrapie prion protein. Nature 375, 698–700 (1995).
Bossers, A. et al. Scrapie susceptivility-linked polymorphisms modulate the in vitro conversion of sheep prion protein to protease-resistant forms. Proc. Natl Acad. Sci. USA 94, 4931–4936 (1997).
Bessen, R. A., Raymond, G. J. & Caughey, B. In situ formation of protease-resistant prion protein in transmissible spongiform encephalopathy-infected brain slices. J. Biol. Chem. 272, 15227–15231 (1997).
Fraser, H., McConnell, I., Wells, G. A. H. & Dawson, M. Transmission of bovine spongiform encephalopathy to mice. Vet. Rec. 123, 472 (1988).
Dawson, M., Wells, G. A. H. & Parker, B. N. J. Preliminary evidence of the experimental transmissibility of bovine spongiform encephalopathy to cattle. Vet. Rec. 126, 112–113 (1990).
Foster, J. D., Hope, J. & Fraser, H. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet. Rec. 133, 339–341 (1993).
Goldmann, W., Hunter, N., Smith, G., Foster, J. & Hope, J. PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie. J. Gen. Virol. 75, 989–995 (1994).
Caughey, B., Kocisko, D. A., Raymond, G. J. & Lansbury, P. T. Aggregates of scrapie associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistant state. Chem. Biol. 2, 807–817 (1995).
Palmer, M. S., Dryden, A. J., Hughes, J. T. & Collinge, J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt–Jakob disease. Nature 352, 340–342 (1991).
Riek, R. et al. NMR structure of the mouse prion protein domain PrP(121–231). Nature 382, 180–182 (1996).
Collinge, J. et al. Unaltered susceptibility to BSE in transgenic mice expressing human prion protein. Nature 378, 779–783 (1995).
Hope, J. et al. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 5, 2591–2597 (1986).
van Keulen, L. J. M. et al. Immunohistochemical detection and localization of prion protein in brain tissue of sheep with natural scrapie. Vet. Pathol. 32, 299–308 (1995).
Kascsak, R. J. et al. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J. Virol. 61, 3688–3693 (1987).
Petersen, R. B., Parchi, P., Richardson, S. L., Urig, C. B. & Gambetti, P. Effect of the D178N mutation and the codon 129 polymorphism on the metabolism of the prion protein. J. Biol. Chem. 271, 12661–12668 (1996).
Caughey, B. et al. in Prion Diseases (eds Baker, H. F. Ridley, R. M.) 285–300 (Humana, Totowa, (1996)).
Caughey, B., Raymond, G. J., Kocisko, D. A. & Lansbury, P. T. J Scrapie infectivity correlates with converting activity, protease resistance, and aggregation of scrapie-associated prion protein in guanidine denaturation studies. J. Virol. 71, 4107–4110 (1997).
Acknowledgements
We thank B. Chesebro, C. Bostock and our colleagues at the Rocky Mountain Laboratories and the Institute for Animal Heath for their suggestions and critiques of this manuscript and our experimental design, and A. Chong for technical assistance, Part of this work was funded by the UK Ministry of Agriculture, Fisheries and Food (J.H.) and by NIH grants and Britton Fund to P.G.
Author information
Authors and Affiliations
Corresponding author
Additional information
Correspondence and requests for materials should be addressed to B.C.
Supplementary Information
Rights and permissions
About this article
Cite this article
Raymond, G., Hope, J., Kocisko, D. et al. Molecular assessment of the potential transmissibilities of BSE and scrapie to humans. Nature 388, 285–288 (1997). https://doi.org/10.1038/40876
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/40876
This article is cited by
-
Current and future applications of induced pluripotent stem cell-based models to study pathological proteins in neurodegenerative disorders
Molecular Psychiatry (2021)
-
Advanced tests for early and accurate diagnosis of Creutzfeldt–Jakob disease
Nature Reviews Neurology (2016)
-
Transmission of scrapie prions to primate after an extended silent incubation period
Scientific Reports (2015)
-
The application of in vitro cell-free conversion systems to human prion diseases
Acta Neuropathologica (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.