Abstract
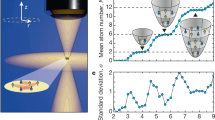
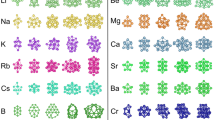
ATOMIC clusters of sodium and other simple metals are known to exhibit a shell structure, giving rise to enhanced stability at certain 'magic numbers' of constituent atoms1–3. Balian and Bloch have shown4 that such shells are the likely result of particularly stable electronic structures: electrons in a spherical cavity (approximating the potential in the clusters) follow semiclassical triangular or square orbits, leading to a shell structure similar to that in atoms5, and stable configurations occur at magic numbers proportional to the cube root of the number of electrons. Balian and Bloch4 also predicted that the existence of both triangular and square orbits, with slightly different periodicities of their magic numbers, should lead to a 'quantum beating' effect that imposes a low-frequency envelope on the periodic variation in cluster stability with increasing size, in effect creating an additional 'supershell' structure. Here we report the observation of this supershell effect in sodium clusters with up to 3,000 constituent atoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Knight, W. D. et al. Phys. Rev. Lett. 52, 2141–2144 (1984).
de Heer, W. A., Knight, W. D., Chou, M. Y. & Cohen, M. L. Solid State Phys. 40, 93–181 (1987).
Katakuse, I. et al. Int. J. Mass Spectrom. Ion Proc. 67, 229–236 (1985).
Balian, R. & Bloch, C. Ann. Phys. 69, 76–160 (1972).
Bjørnholm, S. Contemp. Phys. 31, 309–324 (1990).
Nishioka, H., Hansen, K. & Mottelson, B. R. Phys. Rev. B42, 9377–9386 (1990).
Bjørnholm, S. et al. Phys. Rev. Lett. 65, 1627–1630 (1990).
Martin, T. P., Bergmann, T., Göhlich, H. & Lange, T. Chem. Phys. Lett. 172, 209–213 (1990).
Martin, T. P. et al. Chem. Phys. Lett. (in the press).
Bjørnholm, S. et al. Z. Phys. D19, 47–50 (1991).
Bohr, A. & Mottelson, B. R. Nuclear Structure Vol. II, 608 (Benjamin, London, 1975).
Brack, M., Genzken, O. & Hansen, K. Z. Phys. D21, 65–81 (1991).
Brack, M., Genzken, O. & Hansen, K. Z. Phys. D19, 51–53 (1991).
Brechignac, C., Cahuzac, Ph., Leygnier, J. & Weiner, J. J. chem. Phys. 90, 1492–1498 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pedersen, J., Bjørnholm, S., Borggreen, J. et al. Observation of quantum supershells in clusters of sodium atoms. Nature 353, 733–735 (1991). https://doi.org/10.1038/353733a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/353733a0
This article is cited by
-
Real-time observation of dynamic structure of liquid-vapor interface at nanometer resolution in electron irradiated sodium chloride crystals
Scientific Reports (2020)
-
Statistical and electrical properties of the conduction electrons of a metal nanosphere in the region of metal-insulator transition
Nanoscale Research Letters (2014)
-
Statistical Properties of Conduction Electrons in an Isolated Metal Nanosphere
Journal of Statistical Physics (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.