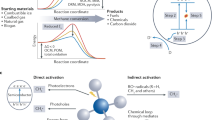
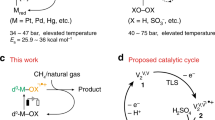
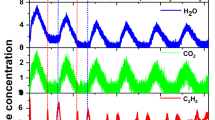
Abstract
The Sabatier reaction: CO2+4H2 → CH4+2H2O(g) ΔG°298K = −27 kcal mol−1 (1) is an important catalytic process of wide industrial and academic interest1—3. It is applied to syngas conversion and the treatment of waste streams. Methane is one of the most important carbon resources of the world, serving as an energy vector as well as a feedstock for higher-value chemicals4—6. Despite its favourable thermodynamics, the eight-electron reduction of CO2 to CH4 by hydrogen is difficult to achieve: high-energy intermediates impose large kinetic barriers, and the formation of side products is common. Intensive investigations during the past decade have therefore been aimed at improving the activity and selectivity of methanation catalysts 1–3,8–11. Although significant progress has been made in this field, elevated temperatures (T>300 °C) and pressures (P> 1 atm) are still required for methane generation to proceed at significant rates and yields. Here we report the selective conversion of CO2 to methane at room temperature and atmospheric pressure, using highly dispersed Ru/RuOx loaded onto TiO2 as a catalyst. The reaction rate is sharply enhanced through photo-excitation of the support material.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Lunde, P. J. & Kester, F. L. / Catal. 30, 423–429 (1973). 2. Phyng Quack, T. Q. & Rouleau, D. /. appl Chem. Biotechnol. 26, 527–535 (1976). 3. Tomsett, A. D., Hagiwara, T., Miyamoto, A. & Inui, T. Appl. Catal. 26, 391–394 (1986). 4. Keim, W. in Chemistry of the Future (ed. Grunewald, H.) 53–62 (Pergamon, Oxford, 1983). 5. Tomoyasu, I. & Lunsford, J. H. Nature 314, 721–722 (1985). 6. Otsuka, K., Jinno, K. & Morikawa, A. /. Catal. 100, 353–359 (1986). 7. Solymosi, R, Erdoheli, A. & Kocsis, M. JCS Faraday Trans. 1 77, 1003–1012 (1981). 8. Solymosi, F., Erdoheli, A. & Bansagi, T. J. Catal. 68, 371–382 (1981). 9. Weatherbee, G. D. & Bartholomew, C. H. /. Catal 87, 352–362 (1984). 10. Inui, T., Funabiki, F., Suehiro, M. & Sezume, T. JCS Faraday Trans. 1 75, 787–802 (1979). 11. Blondeel, G., Harriman, A., Porter, G., Urwin, D. & Kiwi, J. /. phys. Chem. 87, 2629–2636 (1983). 12. Tauster, S. J., Fung, S. C. & Garten, R. L. /. Am. chem. Soc. 100, 170–175 (1978). 13. Anderson, J. R. Structure of Metallic Catalysts 361 (Academic, London, 1973). 14. Gupta, N. M., Kamble, V. S. & lyer, R. M. J. Catal. 66, 101–111 (1980). 15. Yixuan, C. et al. J. molec. Catal. 21, 275–280 (1983). 16. Kawai, T. & Sakata, T. Nature 282, 283–284 (1979). 17. Cunningham, J., Tobin, P. J. & Meriaudeau, P. Surface Sci. 108, L465–L469 (1981). 18. Vijayakumar, K. M. & Lichtin, N. N. / Catal. 90, 173–177 (1984). 19. Cunningham, J. in Comprehensive Chemical Kinetics Vol. 19, Ch. 3 (eds Bamford, C. H., Tipper, C. F. H. & Compton, R.) (Elsevier, Amsterdam, 1984). 20. Wise, H. & McCarthy, J. G. Surface Sci. 133, 311–320 (1983). 21. Frese, K. W. & Leach, S. /. electrochem. Soc. 132, 259–260 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thampi, K., Kiwi, J. & Grätzel, M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature 327, 506–508 (1987). https://doi.org/10.1038/327506a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/327506a0
This article is cited by
-
Probing active sites for carbon oxides hydrogenation on Cu/TiO2 using infrared spectroscopy
Communications Chemistry (2022)
-
The catalytic efficiency of Fe-containing nanocomposites based on highly dispersed silica in the reaction of CO2 hydrogenation
Research on Chemical Intermediates (2022)
-
A Density Functional Theory and Experimental Study of CO2 Photoreduction to Methanol over α-Sulfur-TiO2 Composite
Electrocatalysis (2021)
-
Unlocking the potential of the formate pathway in the photo-assisted Sabatier reaction
Nature Catalysis (2020)
-
MOFs-Based Catalysts Supported Chemical Conversion of CO2
Topics in Current Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.