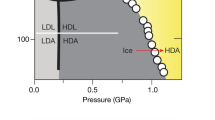
Abstract
Although liquid water has been the focus of intensive research for over 100 years, a coherent physical picture that unifies all of the known anomalies of this liquid1,2,3, is still lacking. Some of these anomalies occur in the supercooled region, and have been rationalized on the grounds of a possible retracing of the liquid–gas spinodal (metastability limit) line into the supercooled liquid region4,5,6,7, or alternatively the presence of a line of first-order liquid–liquid phase transitions in this region which ends in a critical point8,9,10,11,12,13,14,. But these ideas remain untested experimentally, in part because supercooled water can be probed only above the homogeneous nucleation temperature TH at which water spontaneously crystallizes. Here we report an experimental approach that is not restricted by the barrier imposed by TH, involving measurement of the decompression-induced melting curves of several high-pressure phases of ice in small emulsified droplets. We find that the melting curve for ice IV seems to undergo a discontinuity at precisely the location proposed for the line of liquid–liquid phase transitions8. This is consistent with, but does not prove, the coexistence of two different phases of (supercooled) liquid water. From the experimental data we calculate a possible Gibbs potential surface and a corresponding equation of state for water, from the forms of which we estimate the coordinates of the liquid–liquid critical point to be at pressure Pc ≈ 0.1 GPa and temperature Tc ≈ 220 K.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Debenedetti, P. G. Metastable Liquids (Princeton Univ. Press, (1997)).
Angell, C. A. Formation of glasses from liquids and biopolymers. Science 267, 1924–1935 (1995).
Fourkas, J. T., Kivelson, D., Mohanty, U. & Nelson, K. A. (eds) Supercooled Liquids: Advances and Novel Applications (ACS Books, Washington DC, (1997)).
Speedy, R. J. & Angell, C. A. Isothermal compressibility of supercooled water and evidence for a thermodynamic singularity at −45 C. J. Chem. Phys. 65, 851–858 (1976).
Speedy, R. J. Stability-limit conjecture. An interpretation of the properties of water. J. Phys. Chem. 86, 982–991 (1982).
Borick, S. S. & Debenedetti, P. G. Equilibrium, stability and density anomalies in a lattice model with core softening and directional bonding. J. Phys. Chem. 97, 6292–6303 (1993).
Borick, S. S., Debenedetti, P. G. & Sastry, S. Alattice model of network-forming fluids with orientation-dependent bonding: equilibrium, stability, and implications from the phase behavior of supercooled water. J. Phys. Chem. 99, 3781–3793 (1995).
Poole, P. H., Sciortino, F., Essmann, U. & Stanley, H. E. Phase behaviour of metastable water. Nature 360, 324–328 (1992).
Tanaka, H. Aself-consistent phase diagram for supercooled water. Nature 380, 328–331 (1996).
Tanaka, H. Phase behaviors of supercooled water: reconciling a critical point of amorphous ices with spinodal instability. J. Chem. Phys. 105, 5099–5111 (1996).
Roberts, C. J., Panagiotopoulos, A. Z. & Debenedetti, P. G. Liquid-liquid immiscibility in pure fluids: polyamorphism in simulations of a network-forming fluid. Phys. Rev. Lett. 77, 4386–4389 (1996).
Roberts, C. J. & Debenedetti, P. G. Polyamorphism and density anomalies in network-forming fluids: zeroth- and first-order approximations. J. Chem. Phys. 105, 658–672 (1996).
Harrington, S., Zhang, R., Poole, P. H., Sciortino, F. & Stanley, H. E. Liquid-liquid phase transition: Evidence from simulations. Phys. Rev. Lett. 78, 2409–2412 (1997).
Sciortino, F., Poole, P. H., Essmann, U. & Stanley, H. E. Line of compressibility maxima in the phase diagram of supercooled water. Phys. Rev. E 55, 727–737 (1997).
Mishima, O., Calvert, L. D. & Whalley, E. An apparently first-order transition between two amorphous phases of ice induced by pressure. Nature 314, 76–78 (1985).
Mishima, O. Relationship between melting and amorphization of ice. Nature 384, 546–549 (1996).
Bridgman, P. W. Water, in the liquid and five solid forms, under pressure. Proc. Am. Acad. Arts Sci. 47, 441–558 (1911).
Evans, L. F. Selective nucleation of the high pressure ices. J. Appl. Phys. 38, 4930–4932 (1967).
Kanno, H., Speedy, R. & Angell, C. A. Supercooling of water to −92 °C under pressure. Science 189, 880–881 (1975).
Whalley, E., Klug, D. D. & Handa, Y. P. Entropy of amorphous ice. Nature 342, 782–783 (1989).
Mishima, O. Reversible first-order transition between two water amorphs at ∼0.2 GPa and ∼135 K. J. Chem. Phys. 100, 5910–5912 (1994).
Sastry, S., Debenedetti, P., Sciortino, F. & Stanley, H. E. Singularity-free interpretation of the thermodynamics of supercooled water. Phys. Rev. E 53, 6144–6154 (1996).
Fletcher, N. H. The Chemical Physics of Ice (Cambridge Univ. Press, (1970)).
Kamb, B. Structure of ice VI. Science 150, 205–209 (1965).
Speedy, R. J. Thermodynamic properties of supercooled water at 1 atm. J. Phys. Chem. 91, 3354–3358 (1987).
Johari, G. P., Fleissner, G., Hallbrucker, A. & Mayer, E. Thermodynamic continuity between glassy and normal water. J. Phys. Chem. 98, 4719–4725 (1994).
Bellissent-Funel M. C. & Bosio, L. Aneutron scattering study of liquid D2O. J. Chem. Phys. 102, 3727–3735 (1995).
Lang, E. & Ludemann, H.-D. Pressure and temperature dependence of the longitudinal deuterium relaxation times in supercooled heavy water to 300 MPa and 188 K. Ber. Bunsenges. Phys. Chem. 84, 462–470 (1980).
Bridgman, P. W. The pressure-volume-temperature relations of the liquid, and the phase diagram of heavy water. J. Chem. Phys. 3, 597–605 (1935).
Speedy, R. J., Debenedetti, P. G., Smith, R. S., Huang, C. & Kay, B. D. The evaporation rate, free energy, and entropy of amorphous water at 150K. J. Chem. Phys. 105, 240–244 (1996).
Bruggeller, P. & Mayer, E. Complete vitrification in pure liquid water and diute aqueous solutions. Nature 288, 569–571 (1980).
Bellissent-Funel, M. C., Bosio, L., Hallbrucker, A., Mayer, E. & Sridi-Dorbez, R. X-ray and neutron scattering studies of the structure of hyperquenched glassy water. J. Chem. Phys. 97, 1282–1286 (1992).
Haar, L., Gallagher, J. S. & Kell, G. S. NBS/NRC Steam Tables (Hemisphere, Washington DC, (1984)).
Acknowledgements
We thank C. A. Angell, K. Aoki, M.-C. Bellissent-Funel, M. Canpolat, H.-D. Lüdemann, P. H. Poole, R. Sadr-Lahijany, S. Sastry, F. Sciortino, F. W. Starr and Y. Suzuki for discussions and reading of manuscript drafts. We also thank C. A. Angell for pointing out subtle ponts that we initially glossed over. This work was supported by CREST (Core Research for Evolutional Science and Technology) of Japan Science and Technology Corporation (JST), BP and the US NSF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishima, O., Stanley, H. Decompression-induced melting of ice IV and the liquid–liquid transition in water. Nature 392, 164–168 (1998). https://doi.org/10.1038/32386
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/32386
This article is cited by
-
Liquid-liquid phase separation in supercooled water from ultrafast heating of low-density amorphous ice
Nature Communications (2023)
-
Molecular dynamics analysis of elastic properties and new phase formation during amorphous ices transformations
Scientific Reports (2022)
-
Evidence of a liquid–liquid phase transition in H\(_2\)O and D\(_2\)O from path-integral molecular dynamics simulations
Scientific Reports (2022)
-
Fragile to strong crossover and Widom line in supercooled water: A comparative study
Frontiers of Physics (2018)
-
A metastable liquid melted from a crystalline solid under decompression
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.