Abstract
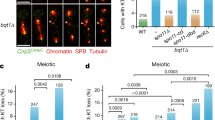
When cells exit from mitotic cell division, their sister chromatids lose cohesion and separate to opposite poles of the dividing cell, resulting in equational chromosome segregation. In contrast, the reductional segregation of the first stage of meiotic cell division (meiosis I) requires that sister chromatids remain associated through their centromeres and move together to the same pole. Centromeric cohesion is lost as cells exit from meiosis II and sister chromatids can then separate1,2,3,4. The fission yeast cohesin protein Rec8 is specific to and required for meiosis5,6,7,8. Here we show that Rec8 appears in the centromeres and adjacent chromosome arms during the pre-meiotic S phase. Centromeric Rec8 persists throughout meiosis I and disappears at anaphase of meiosis II. When the rec8 gene is deleted, sister chromatids separate at meiosis I, resulting in equational rather than reductional chromosome segregation. We propose that the persistence of Rec8 at centromeres during meiosis I maintains sister-chromatid cohesion, and that its presence in the centromere-adjacent regions orients the kinetochores so that sister chromatids move to the same pole. This results in the reductional pattern of chromosome segregation necessary to reduce a diploid zygote to haploid gametes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Miyazaki, W. Y. & Orr-Weaver, T. L. Sister-chromatid cohesion in mitosis and meiosis. Annu. Rev. Genet. 28, 167–168 (1994).
Bickel, S. E. & Orr-Weaver, T. L. Holding chromatids together to ensure they go their separate ways. BioEssays 18, 293–300 (1996).
Kleckner, N. Meiosis: how could it work? Proc. Natl Ac. Sci. USA 93, 8167–8174 (1996).
Roeder, G. S. Meiotic chromosomes: it takes two to tango. Genes Dev. 11, 2600–2621 (1997).
DeVeaux, L. C. & Smith, G. R. Region-specific activators of meiotic recombination in Schizosaccharomyces pompe. Genes Dev. 8, 203–210 (1994).
Molnar, M., Bahler, J., Sipiczki, M. & Kohli, J. The rec8 gene of Schizosaccharomyces pombe is involved in linear element formation, chromosome pairing and sister-chromatid cohesion during meiosis. Genetics 141, 61–73 (1995).
Michaelis, C., Ciosk, R. & Nasmyth, K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91, 35–45 (1997).
Guacci, V., Koshland, D. & Strunnikov, A. Adirect link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 91, 47–57 (1997).
Parisi, S.et al. Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family, conserved from fission yeast to humans. Mol. Cell. Biol. 19, 3515–3528 (1999).
Nabeshima, K.et al. Dynamics of centromeres during metaphase–anaphase transition in fission yeast: dis1 is implicated in force balance in metaphase bipolar spindle. Mol. Biol. Cell 9, 3211–3225 (1998).
Nakaseko, Y., Niwa, O. & Yanagida, M. Ameiotic mutant of the fission yeast Schizosaccharomyces pombe that produces mature asci containing two diploid spores. J. Bacteriorl. 157, 334–336 (1984).
Iino, Y. & Yamamoto, M. Mutants of Schizosaccharomyces pombe which sporulate in the haploid state. Mol. Gen. Genet. 198, 416–421 (1985).
Chikashige, Y.et al. Composite motifs and repeat synmetry in S. pombe centromeres. Cell 57, 739–751 (1989).
Chikashige, Y.et al. Telomere-led premeiotic chromosome movement in fission yeast. Science 264, 270–273 (1994).
Horie, S.et al. The Schizosaccharomyces pombe mei4+ gene encodes a meiosis-specific transcription factor containing a forkhead DNA-binding domain. Mol. Cell. Biol. 18, 2118–2129 (1998).
Kishida, M., Nagai, T., Nakaseko, Y. & Shimoda, C. Meiosis-dependent mRNA splicing of the fission yeast Schizosaccharomyces pombe mes1+ gene. Curr. Genet. 25, 497–503 (1994).
Birkenbihl, R. P. & Subramani, S. The rad21 gene product of Schizosaccharomyces pombe is a nuclear, cell cycle-regulated phosphoprotein. J. Biol. Chem. 270, 7703–7711 (1995).
Toth, A.et al. Yeast Cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev. 13, 320–333 (1999).
Uhlmann, F. & Nasmyth, K. Cohesion between sister chromatids must be established during DNA replication. Curr. Biol. 8, 1095–1101 (1998).
Skibbens, R. V., Corson, L. B., Koshland, D. & Hieter, P. Ctf7p is essential for sister chromatid cohesion and links mitotic chromosome structure to the DNA replication machinery. Genes Dev. 13, 307–319 (1999).
Ciosk, R.et al. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell 93, 1067–1076 (1998).
Tang, T. T., Bickel, S. E., Young, L. M. & Orr-Weaver, T. L. Maintenance of sister-chromatid cohesion at the centromere by the Drosophila MEI-S322 protein. Genes Dev. 12, 3843–3856 (1998).
Moreno, S., Klar, A. & Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 (1991).
Bähler, J.et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 (1998).
Cooper, J. P., Watanabe, Y. & Nurse, P. Fission yeast Taz1 protein is required for meiotic telomere clustering and recombination. Nature 392, 828–831 (1998).
Bähler, J., Wyler, T., Loidl, J. & Kohli, J. Unusual nuclear structures in meiosis prophase of fission yeast: a cytological analysis. J. Cell Biol. 121, 241–256 (1993).
Klein, F.et al. Localization of RAP1 and topoisomerase II in nuclei and meiotic chromosomes of yeast. J. Cell Biol. 117, 9335–948 (1992).
Birkenbihl, R. P. & Subramani, S. Cloning and characterization of rad21 an essential gene of Schizosaccharomyces pombe involved in DNA double-strand-break repair. Nucleic Acids Res. 20, 6605–6611 (1992).
Nomura, N.et al. Prediction of the coding sequences of unidentified human genes. DNA Res. 1, 223–229 (1994).
Acknowledgements
We thank M. Yamamoto, M. Yanagida and S. Subramani for plasmids and strains; J.Bähler, J. Cooper and J. Hayles for advice with the manuscript; and J. Kohli and M. McKay for communicating results before publication. Y.W. thanks all the members of P. Nurse's lab for advice and reagents, and the ICRF staff and M. Yamamoto for support. Y.W. was supported by JSPS and a Uehara fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, Y., Nurse, P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400, 461–464 (1999). https://doi.org/10.1038/22774
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/22774
This article is cited by
-
Promoter hypermethylation and comprehensive regulation of ncRNA lead to the down-regulation of ZNF880, providing a new insight for the therapeutics and research of colorectal cancer
BMC Medical Genomics (2023)
-
ATM signaling modulates cohesin behavior in meiotic prophase and proliferating cells
Nature Structural & Molecular Biology (2023)
-
Learning to tango with four (or more): the molecular basis of adaptation to polyploid meiosis
Plant Reproduction (2023)
-
WDR62 is required for centriole duplication in spermatogenesis and manchette removal in spermiogenesis
Communications Biology (2021)
-
Cloning and characterization of rec8 gene in orange-spotted grouper (Epinephelus coioides) and Dmrt1 regulation of rec8 promoter activity
Fish Physiology and Biochemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.