Abstract
The transport of molecules in confined media is subject to entropic barriers. So theoretically, asymmetry of the confinement length may lead to molecular ratchets with entropy as the only driving force for the biased transport. We address experimentally this question by performing alternative ionic current measurements on electrolytes confined in neutral conical nanopores. In case anions and cations widely differ in size, we show that rectification of ionic current can be obtained that depends on ions size and cycle frequency, consistently with the entropic ratchet mechanism.
Similar content being viewed by others
Introduction
In fluids, due to random motion of molecules, their directed transport is ordinarily achieved by the mean of an external force field. Brownian motors and ratchets1,2,3 fulfill this task without net bias, not only despite random motion but thanks to it. Initially viewed as some thermodynamical curiosities for physicists4,5,6, these brownian machines have gained interest when they proven to be relevant in many biological systems, from muscle contraction7,8 to ionic pumps9, including voltage-gated channels10. Nowadays, advances in nanodevices fabrication stimulate bio-inspired realizations mostly concerning smart ions transport and pumping that likely promises the more challenging applications (e.g. keep in mind water desalination issues).
Ion pumps typically work with nanoporous membranes submitted to a periodic voltage, which is unbiased in average but allows the machines to fulfill the second law of thermodynamics4,5,6. Then, these machines are mostly founded on random motion of ions in a sawtooth landscape of potential that materializes the ratchet. All efforts to produce bio-inspired ionic current rectifiers lie in this materialization11. Until now, concrete realizations12,13,14,15,16,17,18 generate the ratchet by means of electrostatic charges on pore-wall, the asymmetry arises from a charge gradient or from the conical shape of nanopores that is felt by ions through electrostatic interactions19.
In this paper, we consider experimentally a more direct and universal way for any particles to feel the shape of nanopores that could come from entropic barriers20. The idea is the following. For ions with diffusion coefficient D, in solution at concentration c, moving in a potential U, the current J is the sum of brownian diffusion −D∇c and drift motion −Dc∇U/kT:

In confined systems, entropy changes are crucial and the thermodynamic potential to consider in Eq. 1 is more suitably the free energy F = U − TS. The substitution of U by F introduces the entropic forces T∇S. Although these forces do not originate from the microscopic level but rather emerge from the statistics of the entire macroscopic population of particles, they are palpable and have proven to be of great importance in many systems (see e.g. the elasticity of polymer chains21 or the depletion force in colloids22). In a narrow channel, if the transverse equilibrium is rapidly reached, the problem can be treated in 1D using the longitudinal probability density p(x) = c(x)A(x), with A(x) the cross-section area. In ideal solutions, the number of microstates for one given ion is Ω ∝ A(x)/a, with a the area unit. The corresponding entropy is kln(A(x)/a) and Eq. 1 without external potential leads to the Fick-Jacobs equation23:

Eq. 2 is still insufficient to rectify ionic current because charge carriers are dual and the entropic contribution to the free energy is repulsive for both species (contrary to the potential due to pore-wall charge). Thus when transport is facilitated for anions it is impeded for cations and vice versa and the free energy potential remains symmetrical in average. To break the symmetry, the missing piece is related to the depletion layer22: the center of mass of ions cannot approach the wall to distance smaller than their radius r, so the accessible area is rather  24,25. Then, if the pore section is sawtooth, one gets size-dependent entropic barriers that can be used to split ions regarding to their size25. Also, if this idea is correct, thus it should be possible to rectify the ionic current passing through geometrically asymmetric nanopores in case anions and cations widely differ in size (Fig. 1). This is what we experimentally address and report in this paper.
24,25. Then, if the pore section is sawtooth, one gets size-dependent entropic barriers that can be used to split ions regarding to their size25. Also, if this idea is correct, thus it should be possible to rectify the ionic current passing through geometrically asymmetric nanopores in case anions and cations widely differ in size (Fig. 1). This is what we experimentally address and report in this paper.
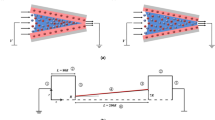
Conical nanopore as entropic rectifier: without transmembrane voltage, the sawtooth free energy potential fsaw is repulsive for both large anions (blue) and small cations (red), but smaller for the latter.
For V > 0, the energy barrier for anions at the pore entrance (tip of cone) is higher than for cations when V < 0. One gets an ionic current diode. In case anions and cations have the same size, the entropic barriers have the same height and no rectification is expected. Here, V(x) stands for the variation of the electric potential on x. For a conical nanopore, this variation is not linear (the electric field is not constant, see Discusion Eq. 6). fsaw does not vary either linearly with x. In this schema, the linear behaviour of the sum of these two contributions is assumed for the sake of simplifying the drawing.
Our experiments consist in ionic current measurements as a function of the applied sinusoidal voltage (cyclic voltammetry) through conical nanopores (track-etched membranes similar to those used in refs 12 and 13) using polyanions as charge carrier (polyacrylic acids, PAA). We show that at pH conditions insuring the electric neutrality of pore-wall, a clear ionic current rectification can be still obtained that depends on polyanions size and cycle frequency.
Experimental details
Membranes with multiple conical nanopores are prepared similarly to ref. 26 by heavy-ion irradiation (Kr28+, 10.4 MeV, fluence 107 cm−2, performed at GANIL, France) of L = 6 μm thick polyimide foils (Kapton HN) and single-side chemical etching of ion-tracks. The etching was performed at 50 °C using a two chambers conductivity cell, one filled with NaOCl-15% etching solution, while the other with 1 M KI neutralizing solution. Transmembrane voltage of +1 V (with respect to the grounded neutralizing compartment) is applied to detect the breakthrough and help the neutralization of reactant as soon as the opening is achieved. Doing so, conical nanopores are obtained with a narrow size distribution, large opening radius B ≃ 200 nm (measured by scanning electron microscopy see Fig. 2) and small opening radius b ≃ 2 nm (estimated from the relation between this size, the conductivity of the electrolyte, and the measured conductance26).
Ionic current was measured using a Biologic-SP200 potentiostat with two Ag/AgCl electrodes. After etching, the two compartments of the conductivity cell were filled with the same electrolyte solution. In order to ensure that the electrode voltage corresponds to the actual transmembrane voltage, the area of the membrane was reduced down to 12.6 mm2 (i.e. 1.26 × 106 pores) allowing its impedance to be larger than 95% of the total (see in Fig. 4 the current drop due to the membrane). Cyclic voltammetry was performed by applying a ±0.5 V sinusoidal voltage (small opening of pores at the grounded compartment) and measuring the current. Cycling was repeated until a stationary behavior (typically 2 or 3 periods). Only these last cycles are shown and discussed in the following. Reversibility was checked after each sample by measuring the membrane behavior with a reference electrolyte (acetic acid 0.1 M).
Results
Dealing with entropic effects on ions transport, the major issue comes from static electric charges on pore-wall that may also lead to rectification of ionic current. Actually, track-etching of polyimide film produces ionized chemical groups at the surface of pore-wall: carboxylic acids and amines. Thus depending on pH, the net charge of pore-wall can be reversed and so the corresponding potential for free-ions14. In Fig. 3A, the ionic current I measured through polyimide conical nanopores is plotted as a function of the membrane voltage V for simple ions (i.e. non-polymeric) solutions at three different pH (KCl 0.1 M in acetate buffer at pH = 2 and 3.6, respectively, and acid acetic 0.1 M pH = 2.8). At pH = 2, carboxylic acids and amines are protonated resulting in a positive net charge of pore-wall, while at pH = 3.6 a sufficient quantity of carboxylic acids are dissociated to allow the inversion of the net charge. This is evidenced by a change of orientation of current rectification (inversion of the concavity of I vs. V). For acetic acid 0.1 M (pH = 2.8), no rectification is observed. These differences are better underlined by using the “normalized rectification efficiency”19,27:
Cyclic voltammetry for conical nanopores filled with KCl 0.1 M solutions at pH = 3.6 and 2, respectively and acetic acid solution (c = 0.1 M, pH = 2.8).
(A) Current I vs. voltage V measured at 1/32 Hz. (B) Rectification efficiency α vs. absolute value of voltage ||V|| (Eq. 3).

For a resistor α = 0, whereas for a diode α = ±1 depending on its direction. Results for α measured for simple ions solutions are plotted in Fig. 3B. We found that at pH = 2.8 ± 0.1, rectification is almost negligible for simple ions solutions. This pH is the isoelectric point of membranes. Results reported below were obtained at this pH, which is naturally obtained with polyacrylic acid (PAA) at a monomer concentration c = 0.1 M, because its dissociation constant (pKa ≃ 4.528) is close to the one of acetic acid. Note that at this concentration only 1.8% of acid groups are dissociated. This is the reason of the low level of the current (typically 1 μA for ≈106 pores, i.e. 1 pA per pore) and justifies the use of multipore-membranes for this study.
Figure 4 shows cyclic voltammetry at 1 Hz for PAA with molecular weight M = 50 × 103 g/mol and acetic acid at the same concentration. Introducing a membrane with conical nanopores between electrodes, causes a current drop but also a patent rectification with PAA as electrolyte, even though the membrane is at its isoelectric point. In Fig. 5, the rectification efficiency α, is plotted as a function of ||V|| for three different PAA molecular weights M and compared to acetic acid. For the two highest molecular weights, the α-values are clearly non-zero and comparable to the values obtained by simulations on other systems19,27. To appreciate this point, one has to keep in mind that for an entropic rectifier of ionic current, the contributions of anions and cations to the conductance add, so that ||α|| < 1 even if the entropic ratchet works perfectly. For instance, point-like ions contributing as a resistor and big counter-ions (with same mobility) contributing as a perfect diode would lead to ||α|| = 0.5. So, here the size dependence of the rectification efficiency is clear. This is our key result.
Cyclic voltammetry at 1 Hz: rectification efficiency α vs. absolute value of voltage ||V|| (Eq. 3) for acetic acid (Ac.Ac., c = 0.1 M) and PAA (𝑀 = 2, 5, and 50 kg/mol, respectively; monomer concentration c = 0.1 M) measured with membrane at the isoelectric point (pH = 2.8 ± 0.1).
Examination of I = f(V) curves (see Figs 3A and 4B), shows that they tend to be linear for large enough ||V|| values. Hence, α(||V||) tends to a maximum αm that can be estimated by:

The ratio αm is plotted versus cycle frequency in Fig. 6 for acetic and PAA. We observe that αm globally decreases for increasing frequency, but also passes by a smooth maximum for the highest molecular weight anion. Also, spectra are shifted to higher frequency for increasing anion size.
Maximum rectification efficiency αm (Eq. 4) vs. cycle frequency ν for acetic acid (Ac.Ac. c = 0.1 M) and PAA of different molecular weights (𝑀 = 2, 5, and 50 kg/mol, respectively; monomer concentration c = 0.1 M) measured with membrane at the isoelectric point (pH = 2.8 ± 0.1).
Discussion
Let us estimate the different characteristic times of our experimental system. The first to consider is the transverse equilibration time that can be identified as the one needed for one chain to diffuse over the large aperture B of nanopore:

This time has to be compared to the time τL needed to cross the channel. Due to the non-constant cross-section area A(x) = πr(x)2 with r(x) = (B − b)/L × x + b, the electric field also is non-constant and writes

For one chain experiencing this field the bias strength is f = E(x)γNe, with e the elementary charge, γ the dissociation ratio of acid groups and N the number of statistical segments per chain (∝ molecular weight). In the stationary stage, this force is balanced by the friction v(x)kT/D, with v(x) the chain velocity, hence

Thus the time τL to cross the entire pore writes:

The last integral is the volume of the entire pore. From measurements reported in literature28, one gets that at low pH the diffusion coefficient D of PAA scales as D = D0 × N−1/2, with D0 ≃ 10−9 m2/s. Note that τB increases for increasing chain length, whereas τL decreases. In Fig. 7, τB and τL were computed from experimental D values28 for V = 0.5 V, L = 6 μm, γ ≃ 2 × 10−2 (i.e. pKa = 4.5 and c = 0.1 M), B = 200 nm and b = 2 nm and plotted as a function of N. One can see that in our experiments τB is always at least 100 times smaller than τL. This gives ground for the 1D treatment at the base of Eq. 2.
Characteristic times of PAA in our conical nanopores vs. number 𝑁 of segments per chain.
τB (dashed red line) is the time needed for a chain to explore radially the pore (Eq. 5). τL (full red line) is the time needed to cross the membrane for a voltage of 0.5 V (Eq. 8). Data points correspond to τB and τL calculated from data of ref. 28. Horizontal dashed lines indicate the time-scale (frequency range) of Fig. 6.
For cycle frequency ν higher than  , polyanions do not have time to cross the entire nanopore and remain localized in a small slice. Hence,
, polyanions do not have time to cross the entire nanopore and remain localized in a small slice. Hence,  would give a simple estimate of the cycle-frequency above which rectification vanishes27. Recent simulations of polyelectrolyte chains translocating through asymmetric nanochannels confort this idea29. This may account for the shift of spectra to high frequency for increasing polyanions size (Fig. 6). Further attempt to rescale data of Fig. 6 by using ντL as reduced variable is at risk, because we still lack the y-axis reduced variable that should be likely necessary. However, it is quite remarkable that the drop of rectification efficiency is found to lie in the time window calculated for τL. This point plainly argues that the energy potential experienced by polyanions extends over the full length L of nanopores and consequently supports the idea of its entropic origin.
would give a simple estimate of the cycle-frequency above which rectification vanishes27. Recent simulations of polyelectrolyte chains translocating through asymmetric nanochannels confort this idea29. This may account for the shift of spectra to high frequency for increasing polyanions size (Fig. 6). Further attempt to rescale data of Fig. 6 by using ντL as reduced variable is at risk, because we still lack the y-axis reduced variable that should be likely necessary. However, it is quite remarkable that the drop of rectification efficiency is found to lie in the time window calculated for τL. This point plainly argues that the energy potential experienced by polyanions extends over the full length L of nanopores and consequently supports the idea of its entropic origin.
Although the membrane is at the isoelectric point, let us consider the only well identified alternative origin of current rectification that should be an electrostatic potential due to the pore-wall. Electrostatic interactions are screened above the Debye’s length λD ≃ 7 nm for cions = γc = 2 × 10−3 M. So, the asymmetric electrostatic potential due to the pore-wall does not extend over the entire pore length but only over the length l = λD × L/(B − b) ≃ 200 nm from the cone tip. As a consequence, in Eq. 8 the volume to consider is  , which is about (L/l)3 = 2 × 104 times smaller than the volume of the entire pore, and also the time needed to cross this small region. This time is out of the time-window of our experiments and could not account for the shift of spectra reported in Fig. 6.
, which is about (L/l)3 = 2 × 104 times smaller than the volume of the entire pore, and also the time needed to cross this small region. This time is out of the time-window of our experiments and could not account for the shift of spectra reported in Fig. 6.
For the three PAA molecular weights here studied, the hydrodynamic radii Rh = kT/(6πηD), with η the viscosity of water, can be computed from the results of ref. 28. They are found to be equal to 1.2, 1.9 and 6 nm, respectively. As regards the pore size (small aperture radius b ≃ 2 nm), the question of chain blockade has to be examined. Actually, the electric field gradient due to the conical shape of pores is responsible for a stretching force applied on chains: fs = γNe × ∇E(x) × R, with R the radius of gyration of chains. This causes chains to elongate longitudinally but also to shrink laterally. In this latter direction, the radius of chain can be estimated as  (see ref. 21 part I.4). Taking R ≃ Rh, one gets the shrinking ratio
(see ref. 21 part I.4). Taking R ≃ Rh, one gets the shrinking ratio  at the cone-tip (maximum field gradient) for the largest PAA chains, which should be sufficient to avoid chain blockades. Note that considering chain deformation does not weaken the previous calculation for τB and τL because deformation is only significant at the cone-tip and the time spent in this region is very short (the field scales as 1/x2 and is maximum at the tip, see Eq. 6) compared to those spent in the rest of the nano-channel, thus this time has a negligible weight in the expression of τL (Eq. 8). Also, note that this argument holds for any phenomenon that could occur at the cone-tip: providing that it does not drastically increase the time spent at the cone-tip (chain blockade) it has practically no incidence on τL and consequently on the measured current.
at the cone-tip (maximum field gradient) for the largest PAA chains, which should be sufficient to avoid chain blockades. Note that considering chain deformation does not weaken the previous calculation for τB and τL because deformation is only significant at the cone-tip and the time spent in this region is very short (the field scales as 1/x2 and is maximum at the tip, see Eq. 6) compared to those spent in the rest of the nano-channel, thus this time has a negligible weight in the expression of τL (Eq. 8). Also, note that this argument holds for any phenomenon that could occur at the cone-tip: providing that it does not drastically increase the time spent at the cone-tip (chain blockade) it has practically no incidence on τL and consequently on the measured current.
Further analysis of our results in relation with theory is not straightforward, especially the non-monotonic rectification spectrum in Fig. 6 that remains quite puzzling. In the literature, equation of motion through entropic barriers was not solved analytically, but with brownian dynamic simulations20,24,25,27. These simulations amount to integrate Langevin equation in some particular cases, which inevitably do not match to our experimental system. In particular, a difference is the coupling between the bias strength and particle size. Until now the case f ∝ R has been considered, whereas f ∝ R2 should be more suitable to fit to our case. May this work stimulates researches in this direction.
Nevertheless, in this paper we have shown that significant rectification of ionic current can be obtained with electrically neutral conical nanopores in case anions and cations widely differ in size. Our observations depend on ions size and cycle frequency. Currently, the entropic ratchet mechanism is the only able to account for them. Also, the entropic ratchet mechanism is possibly relevant for the understanding of facilitated or jammed-like transport observed for ionic-liquids confined in conical nanopores26, with likely outcomes for the improvement of electric batteries and cells that use confined geometries30.
Additional Information
How to cite this article: Lairez, D. et al. The concept of entropic rectifier facing experiments. Sci. Rep. 6, 38966; doi: 10.1038/srep38966 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Astumian, R. D. Thermodynamics and kinetics of a brownian motor. Science 276, 917–922, doi: 10.1126/science.276.5314.917 (1997).
Reimann, P. Brownian motors: noisy transport far from equilibrium. Physics Reports 361, 57–265, doi: 10.1016/S0370-1573(01)00081-3 (2002).
Hänggi, P. & Marchesoni, F. Artificial brownian motors: Controlling transport on the nanoscale. Rev. Mod. Phys. 81, 387–442, doi: 10.1103/RevModPhys.81.387 (2009).
Bridgman, P. W. Note on the principle of detailed balancing. Phys. Rev. 31, 101–102, doi: 10.1103/PhysRev.31.101 (1928).
Brillouin, L. Can the rectifier become a thermodynamical demon? Phys. Rev. 78, 627–628, doi: 10.1103/PhysRev.78.627.2 (1950).
Feynman, R. P., Leighton, R. B. & Sands, M. The Feynman Lectures on Physics: Ratchet and pawl vol. 1, chap. 46 (Addison-Wesley, Reading, MA, 1966).
Vale, R. D. & Oosawa, F. Protein motors and maxwell’s demons: Does mechanochemical transduction involve a thermal ratchet? Adv. Biophys. 26, 97–134 (1990).
Astumian, R. D. & Bier, M. Fluctuation driven ratchets: Molecular motors. Phys. Rev. Lett. 72, 1766–1769, doi: 10.1103/PhysRevLett.72.1766 (1994).
Tsong, T. Y. & Xie, T. D. Ion pump as molecular ratchet and effects of noise: electric activation of cation pumping by na, k-atpase. Appl. Phys. A 75, 345–352, doi: Tsong2002 (2002).
Fadda, G. C., Lairez, D., Guennouni, Z. & Koutsioubas, A. Peptide pores in lipid bilayers: Voltage facilitation pleads for a revised model. Phys. Rev. Lett. 111, 028102-, doi: 10.1103/PhysRevLett.111.028102 (2013).
Hou, X., Zhang, H. & Jiang, L. Building bio-inspired artificial functional nanochannels: From symmetric to asymmetric modification. Angew. Chem. Int. Ed. 51, 5296–5307, doi: 10.1002/anie.201104904 (2012).
Siwy, Z. & Fuliński, A. Fabrication of a synthetic nanopore ion pump. Physical Review Letters 89, 198103-, doi: 10.1103/PhysRevLett.89.198103 (2002).
Siwy, Z. S. Ion-current rectification in nanopores and nanotubes with broken symmetry. Advanced Functional Materials 16, 735–746, doi: 10.1002/adfm.200500471 (2006).
Cervera, J., Schiedt, B., Neumann, R., Mafé, S. & Ramrez, P. Ionic conduction, rectification, and selectivity in single conical nanopores. The Journal of Chemical Physics 124, 104706, doi: 10.1063/1.2179797 (2006).
Sheng, Q., Wang, L., Wang, C., Wang, X. & Xue, J. Fabrication of nanofluidic diodes with polymer nanopores modified by atomic layer deposition. Biomicrofluidics 8, 052111, doi: 10.1063/1.4896474 (2014).
Zhang, Z. et al. Engineered asymmetric heterogeneous membrane: A concentration-gradient-driven energy harvesting device. J. Am. Chem. Soc. 137, 14765–14772, doi: 10.1021/jacs.5b09918 (2015).
Madrid, E. et al. Water desalination concept using an ionic rectifier based on a polymer of intrinsic microporosity (pim). J. Mat. Chem. A 3, 15849–15853, doi: 10.1039/C5TA04092B (2015).
Jiang, X., Liu, Y. & Qiao, R. Current rectification for transport of room-temperature ionic liquids through conical nanopores. J. Phys. Chem. C 120, 4629–4637, doi: 10.1021/acs.jpcc.5b11522 (2016).
Kosińska, I. D., Goychuk, I., Kostur, M., Schmid, G. & Hänggi, P. Rectification in synthetic conical nanopores: A one-dimensional poisson-nernst-planck model. Phys. Rev. E 77, 031131-, doi: 10.1103/PhysRevE.77.031131 (2008).
Reguera, D. et al. Entropic transport: Kinetics, scaling, and control mechanisms. Phys. Rev. Lett. 96, 130603-, doi: 10.1103/PhysRevLett.96.130603 (2006).
de Gennes, P. G. Scaling concepts in polymer physics (Cornell Univ. Press, Ithaca, 1996).
Mao, Y., Cates, M. E. & Lekkerkerker, H. N. W. Depletion force in colloidal systems. Physica A 222, 10–24, doi: 10.1016/0378-4371(95)00206-5 (1995).
Zwanzig, R. Diffusion past an entropy barrier. J. Phys. Chem. 96, 3926–3930, doi: 10.1021/j100189a004 (1992).
Rubí, J. M. & Reguera, D. Thermodynamics and stochastic dynamics of transport in confined media. Chem. Phys. 375, 518–522, doi: 10.1016/j.chemphys.2010.04.029 (2010).
Reguera, D. et al. Entropic splitter for particle separation. Phys. Rev. Lett. 108, 020604-, doi: 10.1103/PhysRevLett.108.020604 (2012).
Tasserit, C., Koutsioubas, A., Lairez, D., Zalczer, G. & Clochard, M. C. Pink noise of ionic conductance through single artificial nanopores revisited. Physical Review Letters 105, 260602-, doi: 10.1103/PhysRevLett.105.260602 (2010).
Motz, T., Schmid, G., Hänggi, P., Reguera, D. & Rub, J. M. Optimizing the performance of the entropic splitter for particle separation. J. Chem. Phys. 141, 074104, doi: 10.1063/1.4892615 (2014).
Swift, T., Swanson, L., Geoghegan, M. & Rimmer, S. The ph-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 12, 2542–2549, doi: 10.1039/C5SM02693H (2016).
Mondal, D. & Muthukumar, M. Ratchet rectification effect on the translocation of a flexible polyelectrolyte chain. J. Chem. Phys. 145, 084906, doi: 10.1063/1.4961505 (2016).
Berrod, Q. et al. Enhanced ionic liquid mobility induced by confinement in 1d cnt membranes. Nanoscale 8, 7845–7848, doi: 10.1039/C6NR01445C (2016).
Author information
Authors and Affiliations
Contributions
D.L. conceived and performed the experiments, analysed the raw data and wrote the manuscript. M.-C.C. prepared the nanoporous membranes. J.-E.W. drawn us attention to this subject. All authors contributed to the understanding of experimental results and to the preparation of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lairez, D., Clochard, MC. & Wegrowe, JE. The concept of entropic rectifier facing experiments. Sci Rep 6, 38966 (2016). https://doi.org/10.1038/srep38966
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38966
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.