Abstract
Considerable controversy exists regarding the association between hyperuricemia and coronary heart disease (CHD). Therefore, we performed a systematic review and dose-response meta-analysis of prospective studies to examine the controversy. Prospective cohort studies with relative risks (RRs) and 95% confidence intervals (CIs) for CHD according to serum uric acid levels in adults were eligible. A random-effects model was used to compute the pooled risk estimate. The search yielded 29 prospective cohort studies (n = 958410 participants). Hyperuricemia was associated with increased risk of CHD morbidity (adjusted RR 1.13; 95% CI 1.05 to 1.21) and mortality (adjusted RR 1.27; 95% CI 1.16 to 1.39). For each increase of 1 mg/dl in uric acid level, the pooled multivariate RR of CHD mortality was 1.13 (95% CI 1.06 to 1.20). Dose-response analysis indicated that the combined RR of CHD mortality for an increase of 1 mg uric acid level per dl was 1.02 (95% CI 0.84 to 1.24) without heterogeneity among males (P = 0.879, I2 = 0%) and 2.44 (95% CI 1.69 to 3.54) without heterogeneity among females (P = 0.526, I2 = 0%). The increased risk of CHD associated with hyperuricemia was consistent across most subgroups. Hyperuricemia may increase the risk of CHD events, particularly CHD mortality in females.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is one of the most common noncommunicable diseases which is forecasted to be the major cause of morbidity and mortality in most developing nations by 20201. In 2011, 375295 Americans died of coronary heart disease (CHD). Each year, an estimated ≈635000 Americans have a new coronary attack and ≈300000 have a recurrent attack. Approximately every 34 seconds, 1 American has a coronary event and approximately every 1 minute 24 seconds, an American will die of one2. The prevention of CVD (especially CHD) is thus clearly a major public health issue. In recent decades, concern has mounted regarding the premature mortality and morbidity associated with CHD, with growing interest in altering risk factors and reversing this global epidemic. Among the novel risk factors for CHD, nutritional factors have aroused particular attention. Prospective observational studies have been used to quantify the total effects of dietary habits on CHD3,4,5. Although the effect of individual components or interactions between dietary habits is still largely unknown or even misconstrue actual total impact on vascular health, elevated serum uric acid levels may explain some of this harmful effect6.
Serum uric acid is a final enzymatic product of purine metabolism7,8. Although hyperuricemia is no universally accepted definition, it is generally defined as serum urate concentration in excess of 6.8 mg/dl9. In the Atherosclerosis Risk in Communities (ARC) Study6, after 3 and 9 years of follow-up, the odds ratio for developing CHD was 2.59 for participants who had a serum acid level >9.0 mg/dl. Moreover, several meta-analyses of observational studies have found that hyperuricemia could significantly increase the risk of CHD events10,11,12. These studies were restricted by heterogeneous with respect to sample size. Furthermore, previous meta-analyses did not assess important differences in the exposure type, such as relevant subgroups (for example, sample size and other characteristics that may contribute to inconsistencies in the literature); and scientific rigour (for example, the quality of the study and duration of follow-up). Additionally, recent studies involving relationship between hyperuricemia and risk of CHD have been published from then on13,14,15,16, results from cohort studies are still controversial. Furthermore, whether any dose-response relation exists between hyperuricemia and risk of CHD is unknown. Therefore, we systematically reviewed and meta-analysed available studies to quantify the associations between hyperuricemia and risk of CHD morbidity and mortality based on identified prospective cohort studies. We also conducted a dose-response analysis for the trend estimation.
Methods
Data Sources and Searches
We performed a systematic search of PubMed (MEDLINE) and EMBASE through August 23, 2015. The following key words were used in our search strategies: (“hyperuricemia” OR “uric acid” OR “urate”) AND (“coronary heart disease” OR “cardiovascular disease” OR “ischemic heart disease” OR “myocardial infarction” OR “coronary artery disease” OR “coronary disease” OR “angina pectoris” OR “unstable angina”) AND (“follow-up studies” OR “prospective studies” OR “cohort studies” OR “longitudinal studies” OR “epidemiological studies” OR “observational studies”). We restricted the search to human studies. No restrictions were imposed on language of publications. In addition, we scrutinized possible eligible references from relevant original papers and review articles to identify potential publications. The search strategy was conducted according to the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) (Appendix 1)17.
Study Selection
Studies were selected for the meta-analysis if they fulfilled the following entry criteria: (1) original studies (eg, not review articles, meeting abstracts, editorials, or commentaries); (2) prospective cohort design (eg, not cross sectional design, case-control design); (3) the exposure of interest was hyperuricemia or elevated serum uric acid level; (4) the outcomes were CHD morbidity and (or) mortality; (5) reported adjusted risk estimates for the association between hyperuricemia assessed as serum and CHD morbidity and/or mortality; (6) longer than one year of follow-up; and (7) participants were free of kidney disease or CHD at study entry. Additionally, we excluded animal studies, clinical trials, commentaries and letters without sufficient data. If data were reported more than once, we included the study with the longest follow-up time. For studies that expressed data separately for males and females, we considered the analysis for each sex as an independent comparison and extracted data separately.
Data Extraction and Quality Assessment
Data were carried out independently by two authors (XZ, XH and WH) using a standard electronic sheets and cross-check to reach a consensus. For each study, the following information was abstracted: name of the first author, year of publication, country where the cohort was performed, geographical location, size of the cohort and proportion of males, age range or mean, duration of follow-up, methods used to assess hyperuricemia and ascertain CHD, number of cases and adjusted covariates. All data were extracted from the published papers. If necessary, the primary authors were contacted to retrieve further information.
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of studies18. The quality of cohort studies were evaluated in the following three major components: selection of the study group (0–4 stars), quality of the adjustment for confounding (0–2 stars) and assessment of outcome in the cohorts (0–3 stars). A higher score represents better methodological quality. The full score was 9 stars. Studies were graded as the high-quality if they met >8 awarded stars.
Data Synthesis and Statistical Analysis
Within each study, we used multivariate-adjusted outcome data (expressed as relative risks [RRs] and 95% confidence intervals [CIs]) for risk estimates. The hazard ratios (HRs) were considered equivalent to RRs, we converted these values in every study by taking their natural logarithms and calculating standard errors and corresponding 95% CIs. RRs and their standard errors were pooled with the DerSimonian and Laird random effects model, which takes into account both within-study and between-study variabilities19. When some studies included in our meta-analysis used the International System (IS) of units to report levels of serum uric acid, we converted those to the conventional units, using a conversion rate of 16.81 (1 mg/dL = 59.48 μmol/L). If the result on CHD was not available, we used data from ischemic heart disease, myocardial infarction or angina pectoris (in the sequential order) as a surrogate for CHD.
For the dose-response analysis, the generalized least square for trend estimation method described by Greenland and Longnecker20 and Orsini et al.21,22 was used to calculate study-specific slopes (linear trends) and 95% CIs. The method requires the distributions of cases and person years for exposure categories and median/mean of serum uric acid levels for each comparison group. We assigned the midpoint of the upper and lower boundaries of each comparison group to determine mean uric acid levels if the median or mean intake was not provided. If the lower or upper boundary for the lowest and highest category, respectively, was not reported, we assumed that the boundary had the same amplitude as the closest category. Additionally, we first created restricted cubic splines with 3 knots at percentiles 25%, 50% and 75% of the distribution23. A P value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the fractional polynomials component is equal to zero.
Heterogeneity among studies was evaluated using the chi-square test based on Cochran’s Q test and I2 statistic at P < 0.10 level of significance19 and quantification of heterogeneity was made by the I2 metric, which describes the percentage of total variation in point estimates that is due to heterogeneity rather than chance24. We considered low, moderate and high degrees of heterogeneity to be I2 values of 25%, 50% and 75%, respectively24,25. To explore possible explanations for heterogeneity and to test the robustness of the association, we conducted subgroup analyses based on the quality of the study (high quality (9) v lower quality (<9), length of follow-up (>10 years v ≤ 10 years), sex (male and female included v female only v male only), number of participants (>5000 v ≤ 5000) and Geographical area (United States versus Asian versus European). Meta-regression and sensitivity analyses were also conducted. We performed the Begg rank correlation test and Egger’s regression test to visualize a possible asymmetry26,27,28. Funnel plots were also used to assess the publication bias. We used a trim and fill algorithm if possible publication bias was detected to identify and correct for the asymmetry of funnel plot from publication bias and provide an adjusted summary RR based on all the studies, including the estimated missing studies29. On the other hand, when the limited number (below 10) of studies was included in each analysis, publication bias was not assessed30.
All the statistical analyses were performed in Stata 12 (Stata Corp, College Station, TX). A threshold of P < 0.1 was used to decide whether heterogeneity or publication bias was present27. In other ways, P values were 2-sided and P < 0.05 was considered statistically significant.
Results
Literature Search
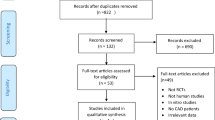
In total the search strategy retrieved 4055 unique articles (1839 articles from PubMed and 2216 articles from EMBASE) (Fig. 1). After exclusion of duplicate records and studies that did not fulfill our inclusion criteria, 107 articles remained and we further evaluated the full texts of these 107 publications. Of these, we excluded 78 studies as follows. Six articles were excluded owing to lack of sufficient data for estimation of RRs. Nine articles were excluded because no original data could be extracted (comment, review, or cross sectional studies). Forty articles were excluded owing to not an outcome of interest. Seventeen articles were excluded because of preexisting CHD at study entry. Three articles were excluded because we deemed irrelevant. Two articles were excluded owing to no adjusted covariates. Another one was excluded because duration of follow-up was shorter than one year. In aggregate, a total of 29 prospective cohort studies representing data from 958410 participants were included in this meta-analysis10,13,14,15,16,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54. In addition, ten studies that expressed data separately for males and females10,15,34,35,37,38,43,45,47,53. According to the study selection criteria, we considered the analysis for each sex as an independent comparison and extracted data separately. Thus, our meta-analysis included 36 comparisons.
Study Characteristics
Table 1 show the characteristics and main outcomes extracted from the included studies, all 29 studies (14 for CHD morbidity10,13,14,31,32,33,34,35,36,37,38,39,40,41 and 15 for CHD mortality15,16,42,43,44,45,46,47,48,49,50,51,52,53,54) were prospective cohort designs and participants who were free of CHD at baseline10,13,14,15,16,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54. In total, the included studies consisted of 958410 participants. Of these participants, we identified 31643 of CHD occurred during follow-up periods ranging from 5.4 to 24.9 years (median of 11.7 years)44,47. Among 29 articles, nine cohorts were conducted primarily in the United States16,31,32,34,35,39,42,43,50, eight articles were done in Asian countries (China, Korea, Israel, Japan)15,37,40,44,46,47,49,54 and twelve cohorts were from European countries (Germany, Italy, Iceland, the Netherlands, Norway, Belgium, Greece, Sweden, Austria)10,13,14,33,36,38,41,45,48,51,52,53. All except one were written in English45. The number of participants ranged from 960 in the Monitoring Trends and Determinants in Cardiovascular Diseases Augsburg (MONICA) cohort by Liese et al.33 to 417734 in the Apolipoprotein MOrtality RISK study (AMORIS) by Holme et al.53. Ten studies included both male and female10,15,34,35,37,38,43,45,47,53, nine studies included only male32,33,39,41,44,46,49,50,51, two cohorts included only female42,52. The age of participants ranged from 20 to 85 years15,53. The definition of hyperuricemia ranged from 5.6 to 7.7 mg/dl in males37,49 and from 4.7 to 7.0 mg/dl in females10,54. In most of the studies, CHD events were assessed by medical records and/or death certificates based on International Classification of Diseases (ICD) codes-8,9,10. All except two studies provided multivariate-adjusted risk estimates (e.g., age, sex, body mass index, smoking, cholesterol, et al.), the majority (93%) of included studies were of high quality (9 stars) (Supplemental table A in appendix 2)32,44.
Hyperuricemia and Risk of CHD Morbidity
18 comparisons from thirteen studies reported an association between hyperuricemia and risk of CHD morbidity, with 6666 CHD cases and 70382 participants10,13,14,32,33,34,35,36,37,38,39,40,41. Overall, the random effects model suggested a positive association; the pooled RR was 1.13 (95% CI 1.05 to 1.21) (Fig. 2). There was moderate study heterogeneity (P = 0.053, I2 = 37.8%). Additionally, slight publication bias was observed from the Begg (P = 0.096), Egger regression tests (P = 0.267) and the funnel plot (see supplemental Figure A in appendix 3). Trim and fill analysis, however, did not change the result (see supplemental Figure B in appendix 3). When a single study involved in the meta-analysis was deleted each time, the results of meta-analysis remained non-significant. A multivariate meta-regression analysis was performed to further investigate the effect of three study-level characteristics (geographical area, sex, length of follow-up and the quality of the study) on the risk of CHD morbidity. None of the regression coefficients was statistically significant (see supplemental table B in appendix 2). Among 18 comparisons, five comparisons (3 for males and 2 for females) were eligible for the dose-response analysis of hyperuricemia and risk of CHD morbidity34,35,41. Using a restricted cubic splines model, we found no evidence of a curve linear association (P = 0.561; P = 0.299 for non-linearity respectively, see supplemental Figures C and D in appendix 3). Dose-response analysis found no associations with risk of CHD morbidity per 1 mg/dl increment of serum uric acid level RR 0.93, 95% CI 0.72 to 1.20, I2 = 34.3%) among males34,35,41 and (RR 1.22, 95% CI 0.83 to 1.80, I2 = 0%) among females (see supplemental Figure E in appendix 3)34,35.
Hyperuricemia and Risk of CHD Mortality
Thirteen studies exported an association between hyperuricemia and risk of CHD mortality, with 24198 CHD cases and 876584 participants15,16,43,44,45,46,47,49,50,51,52,53,54. Using a random effects model summarizing all 18 comparisons, participants with hyperuricemia, compared with normouricemia, experienced a significant increased risk for death of CHD (RR 1.27, 95% CI 1.16 to 1.39) (Fig. 3), although significant heterogeneity was detected (P = 0.000, I2 = 64.9%). The funnel plot was symmetry, no evidence of substantial publication bias was observed from the Begg (P = 0.225) and Egger regression tests (P = 0.102) (see supplemental Figures F and G in appendix 3). When a single study involved in the meta-analysis was also deleted each time, the results of meta-analysis remained non-significant. Moreover, a multivariate meta-regression analysis was also performed to further investigate the effect of four study-level characteristics (geographical area, sex, length of follow-up and the quality of the study) on the risk of CHD mortality. None of the regression coefficients was statistically significant (see supplemental table B in appendix 2). Among 18 comparisons, six comparisons (4 for males and 2 for females) were eligible for the dose-response analysis of hyperuricemia and risk of CHD mortality43,44,46,47,51. Using a restricted cubic splines model, we found no evidence of a curve linear association (P = 0.819 for non-linearity and P for linear trend <0.001 respectively, see supplemental Figures H and I in appendix 3). Dose-response analysis found no associations with risk of CHD mortality per 1 mg/dl increment of serum uric acid level (RR 1.02, 95% CI 0.84 to 1.24, I2 = 0%) among males (see supplemental Figure J in appendix 3)44,46,47,51. However, dose-response analysis found significant association with risk of CHD mortality per 1 mg/dl increment of serum uric acid level (RR 2.44, 95% CI 1.69 to 3.54, I2 = 0%) among females (see supplemental Figure J in appendix 3)43,47.
We paid close attention to each increase of 1 mg/dl in uric acid level. The overall pooled multivariate RR for CHD mortality was 1.15 (N = 5 studies, 95% CI 1.09 to 1.21) (Fig. 4)16,42,43,48,54. The gender specific RRs for each increase of 1 mg/dl in serum uric acid level were similar, but a more pronounced increased risk for CHD mortality in females was observed (Fig. 4). We did not assess publication bias owing to the limited number of included studies.
Subgroup Analyses
To examine the stability of the primary results, we carried out subgroup analyses. The association between hyperuricemia and risk of CHD morbidity and mortality was similar across most subgroups, which were separately defined study quality, length of follow-up, sex, geographical area and number of participants affected the results. The summary estimates of relative risks from each category were pooled (see supplemental table C in appendix 2).
Discussion
The results of this meta-analysis demonstrate that hyperuricemia is prospectively associated with a significantly increased risk of CHD morbidity and mortality. Furthermore, the association persisted and remained statistically significant across most subgroups stratified by various study and participant characteristics. The overall risk of CHD mortality increased 15% for each increase of 1 mg/dl of uric acid, this association seems to be stronger for females that males. Dose-response analyses indicated higher risk of CHD mortality per 1 mg/dl increment of serum uric acid level in females, but no significant trend for males and CHD morbidity. These dose-response results should be interpreted with caution, because the limited number of studies was included in each analysis.
Over the past decades, extensive prospective studies have reported the association of hyperuricemia combined with CVD risk (including CHD and stroke)55,56,57,58,59. However, the role of serum uric acid level in CHD is still controversial. Some of the studies failed to find the association between hyperuricemia and risk of CHD10,13,14,16,32,33,34,35,36,37. However, Chuang and colleagues analysed data from a large Chinese cohort with 7.33 years of follow-up and found that hyperuricemia was independently associated with the development of ischemia heart disease15. Similar to previous analysis in the Apolipoprotein Mortality RISk study (AMORIS), the result from 417734 participants also supported an positive association between hyperuricemia and risk of CHD both in males and in females53. But these studies did not assess or adjust for exercise, education level and other important risk factors such as use of antihypertensive medication48. Diuretics are frequently used to control hypertension and it had been reported to elevate the levels of serum uric acid.
The results from the Framingham Heart Study indicated that uric acid did not have a causal role in the development of CHD, independent of use of diuretics34. However, in the First National Health and Nutrition Examination Survey (NHANES I) Epidemiologic Follow-up Study found that the serum uric acid level was predictive of mortality from ischemic heart disease among females, but no associations were seen among males. These associations were independent of use of antihypertensive agents and diuretics31. Reasons for these conflicting finding were uncertain, but the large intraindividual variation in levels of uric acid, which approaches the variability among persons, may have weakened the strength of the associations observed in several studies60.
Other than hypertension and use of antihypertensive medication or diuretics, age is an important role of other factors such as smoking, alcohol consumption and body mass index. In the Chicago Heart Association Detection Project, CHD deaths were associated with serum uric acid levels in the 55–64 year old group, but not in the 45–54 year group42. But, in the Apolipoprotein MOrtality RISk study (AMORIS), the results supported positive associations between hyperuricemia and risk of CHD. These associations were independent of age53. On the other hand, Freedman et al.31 found that the serum uric acid level was predictive of mortality from ischemic heart disease among females with an age ≥55 years, but no associations were seen among males and patients with an age <55 years.
Several plausible biological mechanisms have been proposed to explain abovementioned association. Uric acid may stimulate vascular smooth cell proliferation, reduce vascular nitric oxide production, diminish vascular nitric oxide activity and link to insulin resistance61,62. It has been demonstrated that uric acid independently associated with the levels of C-reactive protein, interleukin-6, interleukin-18 and tumour necrosis factor-alpha63. On the other hand, inflammation has been considered a key factor during the development of coronary artery disease, via atherogenesis and thrombogenesis64. Moreover, serum uric acid has been positively associated with arterial intima-media thickness, a precursor to atherosclerosis and thus to CHD65. In aggregate, results in metabolic or endocrine changes. Through sympathetic activation and elevated levels of inflammatory cytokines, may link the development of hyperuricemia with the pathogenesis of CHD events.
Heterogeneity between studies was found, which did not alter much in the subgroup analyses. There are differences in serum uric acid levels between males and females among different countries. Therefore, within the subgroup analysis we examined sex and geographical area as possible sources of heterogeneity. Females were more likely than males to have higher prevalence of hypertension and high LDL cholesterol and menopause among females made higher risk than males among in Asian66,67. We also examined study quality, number of participants and length of follow-up as possible sources of heterogeneity, these did not appreciably alter much heterogeneity between studies. Of course, the observed heterogeneity could be attributable to differences in environmental factors, methodological factors in design and how the studies were conducted. Sensitivity analyses showed that exclusion of these only age-adjusted studies did not obviously alter the pooled relative risks32,44. Thus, the presence of heterogeneity calls for caution in interpreting the current meta-analysis findings. However, although the meta-regression could not explain the level of heterogeneity, in interpreting the results, several differences between the studies are worth discussing.
To date, it is generally defined as serum urate concentration exceeding the limit of solubility (about 6.8 mg per deciliter)9. Moreover, it is a common biochemical abnormality that reflects supersaturation of the extracellular fluid with urate and predisposes affected persons to gout. As mentioned earlier, the definition of hyperuricemia ranged from 5.6 to 7.7 mg/dl in males and from 4.7 to 7.0 mg/dl in females. Thus, these ambiguous measurements may underestimate or overestimate true associations between hyperuricemia and risk of coronary artery disease events. Another possible explanation for the differences between the studies might be the classification of outcomes. Coronary artery disease’s definition was inconsistent: the end point from six studies was myocardial infarction14,33,38,39,41,53; four were ischemia heart disease15,31,43,46. If they were included with an uniform definition, the evidences might be stronger.
Compared with the previous meta-analyses10,11,12, our study has several strengths. Our meta-analysis included prospective cohort studies with long duration of follow-up and large sample size, which significantly increased the statistical power to detect potential associations and avoided the influence of recall and selection bias. In addition, to examine the shape of these possible associations, we investigated a dose-response relation hyperuricemia and risk of CHD morbidity and mortality. Furthermore, we used models adjusting for most established risk factors and conducted subgroup analyses and sensitivity analyses and meta-regression to explore whether some characteristics could explain the results and evaluate robustness. Therefore, the results should be more reliable.
There were, however, several limitations of this meta-analysis. Firstly, although in the multivariable analysis we considered a multitude of lifestyle (including exercise) and dietary factors (such as smoking, alcohol). The possibility of residual confounding or confounding by unmeasured factors, which cannot be ruled out in any observational study, must be acknowledged. Second, the noticeable limitation of our study was the potential for bias due to inevitable measurement error, especially for individual with different serum uric acid levels. We attempted to reduce measurement error in adjusting for uric acid levels and using of generally serum urate concentration (6.8 mg/dl). Third, because we had no other sources of information other than medical records and/or death certificates for the identification of CHD events, we might have underestimated the morbidity and/or mortality of CHD. In addition, subclinical diseases at baseline might have distorted our risk estimate to some extent. Finally, the possible limitation is due to language bias. We attempted to minimize this bias by searching major electronic databases with no language restriction.
Conclusions
This meta-analysis provides further evidence that hyperuricemia may increase the risk of CHD events, particularly CHD mortality in females. Future studies, preferably randomized controlled studies of agents that lower or prevent hyperuricemia, should explore whether hyperuricemia is a potentially modifiable risk factor for CHD.
Additional Information
How to cite this article: Li, M. et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci. Rep. 6, 19520; doi: 10.1038/srep19520 (2016).
References
Celermajer, D. S., Chow, C. K., Marijon, E., Anstey, N. M. & Woo, K. S. Cardiovascular disease in the developing world: prevalences, patterns and the potential of early disease detection. J Am Coll Cardiol. 60, 1207–1216 (2012).
Mozaffarian, D. et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 131, e29–322 (2015).
Afshin, A., Micha, R., Khatibzadeh, S. & Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 100, 278–288 (2014).
Mente, A., de Koning, L., Shannon, H. S. & Anand, S. S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 169, 659–669 (2009).
Soedamah-Muthu, S. S. et al. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 93, 158–171 (2011).
Bomback, A. S. et al. Sugar-sweetened soda consumption, hyperuricemia and kidney disease. Kidney Int. 77, 609–616 (2010).
Becker, B. F. Towards the physiological function of uric acid. Free Radic Biol Med. 14, 615–631 (1993).
Sinha, S., Singh, S. N. & Ray, U. S. Total antioxidant status at high altitude in lowlanders and native highlanders: role of uric acid. High Alt Med Biol. 10, 269–274 (2009).
Becker, M. A. et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 353, 2450–2461 (2005).
Wheeler, J. G., Juzwishin, K. D., Eiriksdottir, G., Gudnason, V. & Danesh, J. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: prospective study and meta-analysis. PLoS Med. 2, e76 (2005).
Kim, S. Y. et al. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 62, 170–180 (2010).
Braga, F., Pasqualetti, S., Ferraro, S. & Panteghini, M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med. (2015). 10.1515/cclm-2015-0523.
Kavousi, M. et al. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 156, 438–444 (2012).
Storhaug, H. M. et al. Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: a gender specific analysis from The Tromso Study. BMC Cardiovasc Disord. 13, 115 (2013).
Chuang, S. Y., Chen, J. H., Yeh, W. T., Wu, C. C. & Pan, W. H. Hyperuricemia and increased risk of ischemic heart disease in a large Chinese cohort. Int J Cardiol. 154, 316–321 (2012).
Zalawadiya, S. K. et al. Uric acid and cardiovascular disease risk reclassification: findings from NHANES III. Eur J Prev Cardiol. 22, 513–518 (2015).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 283, 2008–2012 (2000).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 25, 603–605 (2010).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials. 7, 177–188 (1986).
Greenland, S. & Longnecker, M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 135, 1301–1309 (1992).
Berlin, J. A., Longnecker, M. P. & Greenland, S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 4, 218–228 (1993).
Orsini, N., Li, R., Wolk, A., Khudyakov, P. & Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations and software. Am J Epidemiol. 175, 66–73 (2012).
Harrell, F. E. Jr, Lee, K. L. & Pollock, B. G. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 80, 1198–1202 (1988).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ. 327, 557–560 (2003).
Higgins, J. P. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 37, 1158–1160 (2008).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 50, 1088–1101 (1994).
Egger, M., Davey, S. G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 315, 629–634 (1997).
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R. & Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 61, 991–996 (2008).
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 56, 455–463 (2000).
Higgins, J. P. T. & Green, S. Cochrane Handbook for systematic Reviews of Interventions. Oxford, UK: The Cochrane Collaboration; (2011).
Freedman, D. S., Williamson, D. F., Gunter, E. W. & Byers, T. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 141, 637–644 (1995).
Goldberg, R. J. et al. Lifestyle and biologic factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch Intern Med. 155, 686–694 (1995).
Liese, A. D. et al. Association of serum uric acid with all-cause and cardiovascular disease mortality and incident myocardial infarction in the MONICA Augsburg cohort. World Health Organization Monitoring Trends and Determinants in Cardiovascular Diseases. Epidemiology. 10, 391–397 (1999).
Culleton, B. F., Larson, M. G., Kannel, W. B. & Levy, D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 131, 7–13 (1999).
Moriarity, J. T., Folsom, A. R., Iribarren, C., Nieto, F. J. & Rosamond, W. D. Serum uric acid and risk of coronary heart disease: Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 10, 136–143 (2000).
Puddu, P. E. et al. Serum uric acid for short-term prediction of cardiovascular disease incidence in the Gubbio population Study. Acta Cardiol. 56, 243–251 (2001).
Chien, K. L. et al. Hyperuricemia as a risk factor on cardiovascular events in Taiwan: The Chin-Shan Community Cardiovascular Cohort Study. Atherosclerosis. 183, 147–155 (2005).
Bos, M. J., Koudstaal, P. J., Hofman, A., Witteman, J. C. & Breteler, M. M. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 37, 1503–1507 (2006).
Krishnan, E., Baker, J. F., Furst, D. E. & Schumacher, H. R. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 54, 2688–2696 (2006).
Baba, T. et al. Fatty liver and uric acid levels predict incident coronary heart disease but not stroke among atomic bomb survivors in Nagasaki. Hypertens Res. 30, 823–829 (2007).
Meisinger, C., Koenig, W., Baumert, J. & Doring, A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol. 28, 1186–1192 (2008).
Levine, W., Dyer, A. R., Shekelle, R. B., Schoenberger, J. A. & Stamler, J. Serum uric acid and 11.5-year mortality of middle-aged women: findings of the Chicago Heart Association Detection Project in Industry. J Clin Epidemiol. 42, 257–267 (1989).
Fang, J. & Alderman, M. H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 283, 2404–2410 (2000).
Tomita, M. et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 10, 403–409 (2000).
Aboa, E. A. C., De Smet P., Dramaix, M., De Backer, G. & Kornitzer, M. [Relation between uricemia and total, cardiovascular and coronary mortality in both genders of non-selected sample of the Belgium population]. Rev Epidemiol Sante Publique. 49, 531–539 (2001).
Jee, S. H., Lee, S. Y. & Kim, M. T. Serum uric acid and risk of death from cancer, cardiovascular disease or all causes in men. Eur J Cardiovasc Prev Rehabil. 11, 185–191 (2004).
Hakoda, M. et al. Serum uric acid concentration as a risk factor for cardiovascular mortality: a longterm cohort study of atomic bomb survivors. J Rheumatol. 32, 906–912 (2005).
Baibas, N., Trichopoulou, A., Voridis, E. & Trichopoulos, D. Residence in mountainous compared with lowland areas in relation to total and coronary mortality. A study in rural Greece. J Epidemiol Community Health. 59, 274–278 (2005).
Gerber, Y., Tanne, D., Medalie, J. H. & Goldbourt, U. Serum uric acid and long-term mortality from stroke, coronary heart disease and all causes. Eur J Cardiovasc Prev Rehabil. 13, 193–8 (2006).
Krishnan, E., Svendsen, K., Neaton, J. D., Grandits, G. & Kuller, L. H. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 168, 1104–1110 (2008).
Strasak, A. et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clin Chem. 54, 273–284 (2008).
Strasak, A. M. et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. Int J Cardiol. 125, 232–239 (2008).
Holme, I., Aastveit, A. H., Hammar, N., Jungner, I. & Walldius, G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med. 266, 558–570 (2009).
Chen, J. H., Chuang, S. Y., Chen, H. J., Yeh, W. T. & Pan, W. H. Serum uric acid level as an independent risk factor for all-cause, cardiovascular and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. 61, 225–232 (2009).
Dutta, A., Henley, W., Pilling, L. C., Wallace, R. B. & Melzer, D. Uric acid measurement improves prediction of cardiovascular mortality in later life. J Am Geriatr Soc. 61, 319–326 (2013).
Skak-Nielsen, H. et al. Uric acid as a risk factor for cardiovascular disease and mortality in overweight/obese individuals. PLoS One. 8, e59121 (2013).
Savarese, G. et al. Changes in serum uric acid levels and cardiovascular events: a meta-analysis. Nutr Metab Cardiovasc Dis. 23, 707–714 (2013).
Zhao, G., Huang, L., Song, M. & Song, Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. 231, 61–68 (2013).
Li, M., Hou, W., Zhang, X., Hu, L. & Tang, Z. Hyperuricemia and risk of stroke: a systematic review and meta-analysis of prospective studies. Atherosclerosis. 232, 265–270 (2014).
Harris, E. K., Kanofsky, P., Shakarji, G. & Cotlove, E. Biological and analytic components of variation in long-term studies of serum constituents in normal subjects. II. Estimating biological components of variation. Clin Chem. 16, 1022–1027 (1970).
Waring, W. S., Maxwell, S. R. & Webb, D. J. Uric acid concentrations and the mechanisms of cardiovascular disease. Eur Heart J. 23, 1888–1889 (2002).
Dawson, J., Quinn, T. & Walters, M. Uric acid reduction: a new paradigm in the management of cardiovascular risk. Curr Med Chem. 14, 1879–1886 (2007).
Ruggiero, C. et al. Uric acid and inflammatory markers. Eur Heart J. 27, 1174–1181 (2006).
Tiong, A. Y. & Brieger, D. Inflammation and coronary artery disease. Am Heart J. 150, 11–18 (2005).
Montalcini, T. et al. Relation between serum uric acid and carotid intima-media thickness in healthy postmenopausal women. Intern Emerg Med. 2, 19–23 (2007).
Lee, Y. et al. Chin-Shan Community Cardiovascular Cohort in Taiwan-baseline data and five-year follow-up morbidity and mortality. J Clin Epidemiol. 53, 838–846 (2000).
Torng, P. L. et al. Effects of menopause and obesity on lipid profiles in middle-aged Taiwanese women: the Chin-Shan Community Cardiovascular Cohort Study. Atherosclerosis. 153, 413–421 (2000).
Acknowledgements
The authors thank Zhuo Liu, MS, for help with manuscript preparation.
Author information
Authors and Affiliations
Contributions
M.L. and Z.T. conceived and designed the study. M.L. and Y.F. searched the databases and checked them according to the eligible criteria and exclusion criteria. Z.T. helped develop search strategies. X.Z., X.H. and W.H. extract quantitative data. Y.F., X.Z., K.L. and W.H. analyzed the data. M.L. wrote the draft of the paper. All authors contributed to writing, reviewing, or revising the paper. Z.T. is the guarantor.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, M., Hu, X., Fan, Y. et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep 6, 19520 (2016). https://doi.org/10.1038/srep19520
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19520
This article is cited by
-
Predictive value of the serum uric acid to high-density lipoprotein cholesterol ratio for culprit plaques in patients with acute coronary syndrome
BMC Cardiovascular Disorders (2024)
-
Effects of uric acid on ischemic diseases, stratified by lipid levels: a drug-target, nonlinear Mendelian randomization study
Scientific Reports (2024)
-
Association between HMGCR, CRP, and CETP gene polymorphisms and metabolic/inflammatory serum profile in healthy adolescents
Journal of Translational Medicine (2023)
-
Association of hyperuricemia combined with sarcopenia on ASCVD risk
BMC Cardiovascular Disorders (2023)
-
The synergistic effect of the triglyceride-glucose index and serum uric acid on the prediction of major adverse cardiovascular events after coronary artery bypass grafting: a multicenter retrospective cohort study
Cardiovascular Diabetology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.