Abstract
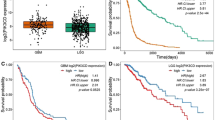
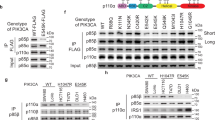
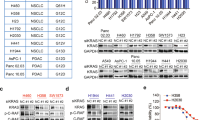
PIKE-A (phosphoinositide 3-kinases (PI 3)-kinase enhancer) is a ubiquitously expressed GTPase, which binds to and enhances protein kinase B (Akt) kinase activity in a guanine nucleotide-dependent manner. PIKE-A is one of the components of the CDK4 amplicon that is amplified in numerous human cancers. However, whether PIKE-A itself can mediate cell transformation, proliferation and migration remains unknown. Here, we show that PIKE-A is overexpressed in various human cancer samples, escalates U87MG glioblastoma invasion and provokes NIH3T3 cell transformation. Overexpression of wild-type (WT) PIKE-A enhances NIH3T3 and U87MG cell growth, which is further increased by cancer cell-derived PIKE-A active mutants. In contrast, both the dominant-negative mutant and the phosphoinositide lipids interaction-defective mutant antagonize cell proliferation. Moreover, PIKE-A and its active and inactive mutants similarly enhance or antagonize U87MG cell survival and invasion, and their ability to do so is coupled with the catalytic effect they have on Akt activation. Furthermore, PIKE-A WT and its active mutants significantly elicit NIH3T3 cell transformation. Thus, our findings support the concept that PIKE-A acts as a proto-oncogene, promoting cell transformation through Akt activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ahn JY, Hu Y, Kroll TG, Allard P, Ye K . (2004a). PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc Natl Acad Sci USA 101: 6993–6998.
Ahn JY, Rong R, Kroll TG, Van Meir EG, Snyder SH, Ye K . (2004b). PIKE (Phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J Biol Chem 279: 16441–16451.
Ahn JY, Rong R, Liu X, Ye K . (2004c). PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J 23: 3995–4006.
Bellacosa A, Testa JR, Staal SP, Tsichlis PN . (1991). A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254: 274–277.
Brazil DP, Park J, Hemmings BA . (2002). PKB binding proteins. Getting in on the Akt. Cell 111: 293–303.
Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC et al. (1992). AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA 89: 9267–9271.
Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK et al. (1996). Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA 93: 3636–3641.
Coffer PJ, Jin J, Woodgett JR . (1998). Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J 335 (Part 1): 1–13.
Collins VP . (1995). Gene amplification in human gliomas. Glia 15: 289–296.
Cox AD, Der CJ . (1994). Biological assays for cellular transformation. Methods Enzymol 238: 277–294.
Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR et al. (1999). Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem 274: 30101–30108.
Hill MM, Hemmings BA . (2002). Inhibition of protein kinase B/Akt: Implication for cancer therapy. Pharmacol Ther 93: 243–251.
Hu Y, Liu Z, Ye K . (2005). Phosphoinositol lipids bind to phosphatidylinositol 3 (PI3) kinase enhancer GTPase and mediate its stimulatory effect on PI3-kinase and Akt signalings. Proc Natl Acad Sci USA 102: 16853–16858.
Huang ZY, Baldwin RL, Hedrick NM, Gutmann DH . (2002). Astrocyte-specific expression of CDK4 is not sufficient for tumor formation, but cooperates with p53 heterozygosity to provide a growth advantage for astrocytes in vivo. Oncogene 21: 1325–1334.
Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS et al. (2001). Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell 8: 693–704.
Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA . (1999). Identification of a human Akt3 (protein kinase B gamma) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Commun 257: 906–910.
Nicholson KM, Anderson NG . (2002). The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 14: 381–395.
Ortega S, Malumbres M, Barbacid M . (2002). Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 1602: 73–87.
Park BK, Zeng X, Glazer RI . (2001). Akt1 induces extracellular matrix invasion and matrix matalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res 61: 7647–7653.
Reifenberger G, Reifenberger J, Ichimura K, Collins VP . (1995). Amplification at 12q13–14 in human malignant gliomas is frequently accompanied by loss of heterozygosity at loci proximal and distal to the amplification site. Cancer Res 55: 731–734.
Reifenberger G, Reifenberger J, Ichimura K, Meltzer PS, Collins VP . (1994). Amplification of multiple genes from chromosomal region 12q13–14 in human malignant gliomas: Preliminary mapping of the amplicons shows preferential involvement of CDK4, SAS, and MDM2. Cancer Res 54: 4299–4303.
Rempel SA, Golembieski WA, Fisher JL, Maile M, Nakeff A . (2001). SPARC modulates cell growth, attachment and migration of U87 glioma cells on brain extracellular matrix proteins. J Neurooncol 53: 149–160.
Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA et al. (2003). PI3 kinase enhancer-Homer complex couples mGIuRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci 6: 1153–1161.
Smith SH, Weiss SW, Jankowski SA, Coccia MA, Meltzer PS . (1992). SAS amplification in soft tissue sarcomas. Cancer Res 52: 3746–3749.
Staal SP . (1987). Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA 84: 5034–5037.
Ye K . (2005). PIKE/nuclear PI 3-kinase signaling in preventing programmed cell death. J Cell Biochem 96: 463–472.
Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ et al. (2002). Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415: 541–544.
Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ et al. (2000). Pike. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell 103: 919–930.
Acknowledgements
This work is supported by grants from NIH (RO1, NS045627) and The Sontag Foundation to K Ye.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Hu, Y., Hao, C. et al. PIKE-A is a proto-oncogene promoting cell growth, transformation and invasion. Oncogene 26, 4918–4927 (2007). https://doi.org/10.1038/sj.onc.1210290
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210290
Keywords
This article is cited by
-
SP1 and RARα regulate AGAP2 expression in cancer
Scientific Reports (2019)
-
Co-amplification of phosphoinositide 3-kinase enhancer A and cyclin-dependent kinase 4 triggers glioblastoma progression
Oncogene (2017)
-
Fyn-phosphorylated PIKE-A binds and inhibits AMPK signaling, blocking its tumor suppressive activity
Cell Death & Differentiation (2016)
-
The roles of PIKE in tumorigenesis
Acta Pharmacologica Sinica (2013)
-
Disrupting the PIKE-A/Akt interaction inhibits glioblastoma cell survival, migration, invasion and colony formation
Oncogene (2013)