Abstract
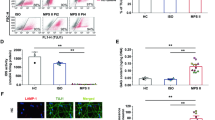
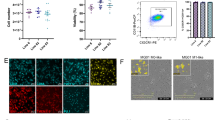
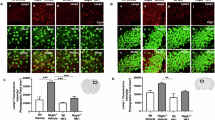
Mucopolysaccharidoses (MPS) are inherited, severe, progressive, metabolic disorders caused by deficiencies in different enzymes involved in degradation of glycosaminoglycans (GAGs). Although enzyme replacement therapy (ERT) has recently been available for MPS type I, and clinical trials have been performed in ERT for MPS II and MPS VI, there is little chance that this kind of treatment may be effective for neurodegenerative forms of MPS (due to inefficient delivery of enzymes to central nervous system through the blood–brain barrier), hence currently there is no effective therapy available for them. Therefore, we aim to develop an alternative therapy for these diseases. We found that genistein (4′,5,7-trihydroxyisoflavone or 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) inhibits synthesis of GAGs considerably in cultures of fibroblasts of MPS patients (types I, II, IIIA and IIIB were tested). Prolonged cultivation of these cells in the presence of genistein resulted in reduction of GAG accumulation and normalization of cells as estimated by biochemical tests and electron microscopic analysis, respectively. As genistein inhibits kinase activity of epidermal growth factor receptor, which is required for full expression of genes coding for enzymes involved in GAG production, we propose to consider a substrate reduction therapy for MPS, which is referred to as ‘gene expression-targeted isoflavone therapy’.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Raman R, Sasisekharan V, Sasisekharan R : Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol 2005; 12: 267–277.
Neufeld EF, Muenzer J : The mucopolysaccharidoses; in Scriver CR, Beaudet AL, Sly WS, Valle D (eds):: The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill Co, 2001, pp 3421–3452.
Węgrzyn G, Węgrzyn A, Tylki-Szymańska A : A general model for genetic regulation of turnover of glycosaminoglycans suggests a possible procedure for prediction of severity and clinical progress of mucopolysaccharidoses. Med Hypoth 2004; 62: 986–992.
Kakkis ED, Muenzer J, Tiller GE et al: Enzyme-replacement therapy in mucopolysaccharidosis I. New Engl J Med 2001; 344: 182–188.
Wraith JE, Clarke LA, Beck M et al: Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human α-L-iduronidase (Laronidase). J Pediatr 2004; 144: 581–588.
Wraith JE : The first 5 years of clinical experience with laronidase enzyme replacement therapy for mucopolysaccharidosis I. Expert Opin Pharmacother 2005; 6: 489–506.
Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA : Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta Paediatr Suppl 2002; 91: 98–99.
Harmatz P, Whitley CB, Waber L et al: Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux–Lamy syndrome). J Pediatr 2004; 144: 574–580.
Cox TM : Substrate reduction therapy for lysosomal storage diseases. Acta Pediatr Suppl 2005; 94: 69–75.
Sato T, Gotoh M, Kiyohara K et al: Differential roles of two N-acetylgalactosaminyltransferases, CSGalNAcT-1, and a novel enzyme, CSGalNAcT-2. J Biol Chem 2003; 278: 3063–3071.
Brown A, Jolly P, Wai H : Genistein modulates neuroblastoma cell proliferation and differentiation through induction of apoptosis and regulation of tyrosine kinase activity and N-myc expression. Carcinogenesis 1998; 19: 991–997.
Murata M, Bonassar LJ, Wright M, Mankin HJ, Towle CA : A role for the interleukin-1 receptor in the pathway linking static mechanical compression to decreased proteoglycan synthesis in surface articular cartilage. Arch Biochem Biophys 2003; 413: 229–235.
Bartold PM, Moule AJ, Li H, Rigby P : Isolation and characterization of the proteoglycans synthesized by adult human pulp fibroblasts in vitro. Int Endod J 1995; 28: 163–171.
Barbosa I, Garcia S, Barbier-Chassefiere V, Caruelle JP, Martelly I, Papy-Garcia D : Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology 2003; 13: 647–653.
Paradisi M, McClintock D, Boguslavsky RL, Pedicelli C, Worman HJ, Djabali K : Dermal fibroblasts in Hutchinson–Gilford progeria syndrome with the lamin A G608G mutation have dysmorphic nuclei and are hypersensitive to heat stress. BMC Cell Biol 2005; 6: 27.
Pisano MM, Greene RM : Epidermal growth factor potentiates the induction of ornithine decarboxylase activity by prostaglandins in embryonic palate mesenchymal cells: effects on cell proliferation and glycosaminoglycan synthesis. Dev Biol 1987; 122: 419–431.
Tirone E, D'Alessandris C, Hascall VC, Siracusa G, Salustri A : Hyaluronan synthesis by mouse cumulus cells is regulated by interactions between folicle-stimulating hormone (or epidermal growth factor) and a soluble oocyte factor (or transforming growth factor β1). J Biol Chem 1997; 272: 4787–4794.
Akiyama T, Ishida J, Nakagawa S et al: Genistein, a specific inhibitor of tyrosine-specific protein kinase. J Biol Chem 1987; 262: 5592–5595.
Kim H, Peterson TG, Barnes S : Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am J Clin Nutr 1998; 68: 1418S–1425S.
Tzanakakis GN, Hjerpe A, Karamanos NK : Proteoglycan synthesis induced by transforming and basic fibroblast growth factors in human malignant mesothelioma is mediated through specific receptors and the tyrosine kinase intracellular pathway. Biochimie 1997; 79: 323–332.
Mitropoulou TN, Tzanakakis GN, Nikitovic D, Tsatsakis A, Karamanos NK : In vitro effects of genistein on the synthesis and distribution of glycosaminoglycans/proteoglycans by estrogen receptor-positive and -negative human breast cancer epithelial cells. Anticancer Res 2002; 22: 2841–2846.
Nikitovic D, Tsatsakis AM, Karamanos NK, Tzanakakis GN : The effects of genistein on the synthesis and distribution of glycosaminoglycans/proteoglycans by two osteosarcoma cell lines depends on tyrosine kinase and the estrogen receptor density. Anticancer Res 2003; 23: 459–464.
Kato M, Takeda S, Ogawara S, Takayama S : Effect of levofloxacin on glycosaminoglycan and DNA synthesis of cultured rabbit chondrocytes at concentrations inducing cartilage lesions in vivo. Antimicrob Agents Chemother 1995; 39: 1979–1983.
Kloska A, Bohdanowicz J, Konopa G et al: Changes in hair morphology of mucopolysaccharidosis I patients treated with recombinant human α-l-iduronidase (laronidase, Aldurazyme). Am J Med Genet 2005; 139A: 199–203.
Węgrzyn G, Kurlenda J, Liberek A et al: Atypical microbial infections of digestive tract may contribute to diarrhea in mucopolysaccharidosis patients: a MPS I case study. BMC Pediatr 2005; 5: 9.
Tsai TH : Concurrent measurement of unbound genistein in the blood, brain and bile of anesthetized rats using microdialysis and its pharmacokinetic application. J Chromatogr A 2005; 1073: 317–322.
Kakkis E, McEntee M, Vogler C et al: Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol Genet Metab 2004; 83: 163–174.
Vogler C, Levy B, Grubb JH et al: Overcoming the blood–brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA 2005; 102: 14777–14782.
Bloedon LT, Jeffcoat AR, Lopaczynski W et al: Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr 2002; 76: 1126–1137.
Busby MG, Jeffcoat AR, Bloedon LT et al: Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr 2002; 75: 126–136.
McClain RM, Wolz E, Davidovich A, Pfannkuch F, Bausch J : Subchronic and chronic safety studies with genistein in dogs. Food Chem Toxicol 2005; 43: 1461–1482.
Ullmann U, Metzner J, Frank T, Cohn W, Riegger C : Safety, tolerability, and pharmacokinetics of single ascending doses of synthetic genistein (Bonistein) in healthy volunteers. Adv Ther 2005; 22: 65–78.
Fischer L, Mahoney C, Jeffcoat AR et al: Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer 2004; 48: 160–170.
Barnes S : Soy isoflavones – phytoestrogens and what else? J Nutr 2004; 134: 1225S–1228S.
Giampietro PG, Bruno G, Furcolo G et al: Soy protein formulas in children: no hormonal effects in long-term feeding. J Pediatr Endocrinol Metab 2004; 17: 191–196.
Chen A, Rogan WJ : Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Annu Rev Nutr 2004; 24: 33–54.
McClain RM, Wolz E, Davidovich A, Bausch J : Genetic toxicity studies with genistein. Food Chem Toxicol 2006; 44: 42–55.
Acknowledgements
We are grateful to Jerzy Bohdanowicz for his help and advice in electron microscopic analyses. This work was funded by the British Society for Mucopolyssacharide Diseases (project no. J4G/25/04), Daniels Sanfilippo Fund (Grant no. DSF-UG-01/2005) and Polish Ministry of Education and Science (project no. 2 P05A 103 26). The use of genistein in treatment of mucopolysaccharidoses is a subject of patent application (application no. P-377180).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piotrowska, E., Jakóbkiewicz-Banecka, J., Barańska, S. et al. Genistein-mediated inhibition of glycosaminoglycan synthesis as a basis for gene expression-targeted isoflavone therapy for mucopolysaccharidoses. Eur J Hum Genet 14, 846–852 (2006). https://doi.org/10.1038/sj.ejhg.5201623
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.ejhg.5201623
Keywords
This article is cited by
-
Activities of (Poly)phenolic Antioxidants and Other Natural Autophagy Modulators in the Treatment of Sanfilippo Disease: Remarkable Efficacy of Resveratrol in Cellular and Animal Models
Neurotherapeutics (2023)
-
Cardiac involvement in MPS patients: incidence and response to therapy in an Italian multicentre study
Orphanet Journal of Rare Diseases (2022)
-
Mucopolysaccharidosis and Autophagy: Controversies on the Contribution of the Process to the Pathogenesis and Possible Therapeutic Applications
NeuroMolecular Medicine (2020)
-
New treatments for the mucopolysaccharidoses: from pathophysiology to therapy
Italian Journal of Pediatrics (2018)
-
EGFR activation triggers cellular hypertrophy and lysosomal disease in NAGLU-depleted cardiomyoblasts, mimicking the hallmarks of mucopolysaccharidosis IIIB
Cell Death & Disease (2018)