Abstract
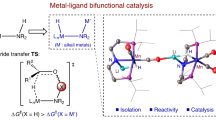
Noyori-type catalysts have found numerous applications in research and industrial settings. The central mechanistic component of such catalysts is a metal centre coordinated to a N–H moiety. This amino moiety has traditionally been thought to participate directly in catalytic reactions by serving as a H+ donor, with the resulting amido group then serving as a H+ acceptor. This traditional understanding has been supplanted by more recent studies that instead suggest that the N–H group(s) (or N–Ma group(s) obtained in the reaction with a base of an alkali metal Ma) serve to stabilize rate-determining transition states through non-covalent N–X···O interactions (X = H or Ma). Thus, N–X bonds are actually not cleaved or formed in many catalytic cycles. This Review describes examples of metal–ligand bifunctional catalysts relevant to reactions involving H2 or its equivalents, emphasizing systems that have been applied in industry. Subsequently, a summary of our present understanding of the Noyori–Ikariya and Noyori reaction mechanisms is presented, which we compare to topical related reactions such as MeOH dehydrogenation, ester and carboxamide hydrogenation and the dehydrogenative coupling of primary alcohols with other alcohols and amines. This mechanistic understanding allows us to identify the design principles that may potentially afford improved molecular catalysts and that may unravel a distinct mechanism for H2 production by the diiron hydrogenase enzymes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Temkin, O. N. Homogeneous Catalysis with Metal Complexes: Kinetic Aspects and Mechanisms. (Wiley-VCH, Weinheim, 2012).
Gridnev, I. D. & Dub, P. A. Enantioselection in Asymmetric Catalysis. (CRC Press, Boca Raton, 2016).
Gridnev, I. D. & Imamoto, T. Enantioselection mechanism in Rh-catalyzed asymmetric hydrogenation. Russ. Chem. Bull. 65, 1514–1534 (2016).
Gridnev, I. D. & Imamoto, T. Mechanism of enantioselection in Rh-catalyzed asymmetric hydrogenation. The origin of utmost catalytic performance. Chem. Commun. 7447–7464 (2009).
Dub, P. A. & Gordon, J. C. The mechanism of enantioselective ketone reduction with Noyori and Noyori–Ikariya bifunctional catalysts. Dalton Trans. 45, 6756–6781 (2016).
Ohkuma, T. et al. Asymmetric hydrogenation of alkenyl, cyclopropyl, and aryl ketones. RuCl2(xylbinap)(1,2-diamine) as a precatalyst exhibiting a wide scope. J. Am. Chem. Soc. 120, 13529–13530 (1998).
Ohkuma, T. et al. Asymmetric hydrogenation of tert-alkyl ketones. J. Am. Chem. Soc. 127, 8288–8289 (2005).
Matsumura, K. et al. Chiral ruthenabicyclic complexes: precatalysts for rapid, enantioselective, and wide-scope hydrogenation of ketones. J. Am. Chem. Soc. 133, 10696–10699 (2011).
Kozuch, S. & Martin, J. M. L. “Turning over” definitions in catalytic cycles. ACS Catal. 2, 2787–2794 (2012).
Ohkuma, T., Ooka, H., Hashiguchi, S., Ikariya, T. & Noyori, R. Practical enantioselective hydrogenation of aromatic ketones. J. Am. Chem. Soc. 117, 2675–2676 (1995).
Hashiguchi, S., Fujii, A., Takehara, J., Ikariya, T. & Noyori, R. Asymmetric transfer hydrogenation of aromatic ketones catalyzed by chiral ruthenium(ii) complexes. J. Am. Chem. Soc. 117, 7562–7563 (1995).
Fujii, A., Hashiguchi, S., Uematsu, N., Ikariya, T. & Noyori, R. Ruthenium(ii)-catalyzed asymmetric transfer hydrogenation of ketones using a formic acid–triethylamine mixture. J. Am. Chem. Soc. 118, 2521–2522 (1996).
Uematsu, N., Fujii, A., Hashiguchi, S., Ikariya, T. & Noyori, R. Asymmetric transfer hydrogenation of imines. J. Am. Chem. Soc. 118, 4916–4917 (1996).
Murata, Y. & Torihara, M. WO2014203963A1 (2014).
Kuriyama, W. et al. Catalytic hydrogenation of esters. Development of an efficient catalyst and processes for synthesising (R)-1,2-propanediol and 2-(L-menthoxy)ethanol. Org. Process Res. Dev. 16, 166–171 (2012).
Touge, T. et al. Oxo-tethered ruthenium(ii) complex as a bifunctional catalyst for asymmetric transfer hydrogenation and H2 hydrogenation. J. Am. Chem. Soc. 133, 14960–14963 (2011).
Saudan, L. A., Saudan, C. M., Debieux, C. & Wyss, P. Dihydrogen reduction of carboxylic esters to alcohols under the catalysis of homogeneous ruthenium complexes: high efficiency and unprecedented chemoselectivity. Angew. Chem. Int. Ed. 46, 7473–7476 (2007).
Abdur-Rashid, K. WO2004096735A2 (2004).
Elangovan, S. et al. Selective catalytic hydrogenations of nitriles, ketones, and aldehydes by well-defined manganese pincer complexes. J. Am. Chem. Soc. 138, 8809–8814 (2016).
Wu, W. et al. Iridium catalysts with f-amphox ligands: asymmetric hydrogenation of simple ketones. Org. Lett. 18, 2938–2941 (2016).
Bigler, R. & Mezzetti, A. Highly enantioselective transfer hydrogenation of polar double bonds by macrocyclic iron(ii)/(NH)2P2 catalysts. Org. Process Res. Dev. 20, 253–261 (2016).
Zuo, W. & Morris, R. H. Synthesis and use of an asymmetric transfer hydrogenation catalyst based on iron(ii) for the synthesis of enantioenriched alcohols and amines. Nat. Protoc. 10, 241–257 (2015).
Tan, X. et al. Highly efficient tetradentate ruthenium catalyst for ester reduction: especially for hydrogenation of fatty acid esters. Org. Lett. 17, 454–457 (2015).
Mukherjee, A., Srimani, D., Chakraborty, S., Ben-David, Y. & Milstein, D. Selective hydrogenation of nitriles to primary amines catalyzed by a cobalt pincer complex. J. Am. Chem. Soc. 137, 8888–8891 (2015).
Filonenko, G. A. et al. Bis-N-heterocyclic carbene aminopincer ligands enable high activity in Ru-catalyzed ester hydrogenation. J. Am. Chem. Soc. 137, 7620–7623 (2015).
Spasyuk, D., Vicent, C. & Gusev, D. G. Chemoselective hydrogenation of carbonyl compounds and acceptorless dehydrogenative coupling of alcohols. J. Am. Chem. Soc. 137, 3743–3746 (2015).
Spasyuk, D., Smith, S. & Gusev, D. G. Replacing phosphorus with sulfur for the efficient hydrogenation of esters. Angew. Chem. Int. Ed. 52, 2538–2542 (2013).
Zuo, W., Lough, A. J., Li, Y. F. & Morris, R. H. Amine(imine)diphosphine iron catalysts for asymmetric transfer hydrogenation of ketones and imines. Science 342, 1080–1083 (2013).
Xie, J.-H., Liu, X.-Y., Xie, J.-B., Wang, L.-X. & Zhou, Q.-L. An additional coordination group leads to extremely efficient chiral iridium catalysts for asymmetric hydrogenation of ketones. Angew. Chem. Int. Ed. 50, 7329–7332 (2011).
Zweifel, T., Naubron, J.-V., Büttner, T., Ott, T. & Grützmacher, H. Ethanol as hydrogen donor: highly efficient transfer hydrogenations with rhodium(i) amides. Angew. Chem. Int. Ed. 47, 3245–3249 (2008).
Dub, P. A., Scott, B. L. & Gordon, J. C. Air-stable NNS (ENENES) ligands and their well-defined ruthenium and iridium complexes for molecular catalysis. Organometallics 34, 4464–4479 (2015).
Dub, P. A. & Gordon, J. C. WO2015191505A1 (2015).
Liu, T. et al. Iron complexes bearing diphosphine ligands with positioned pendant amines as electrocatalysts for the oxidation of H2. Organometallics 34, 2747–2764 (2015).
Hulley, E. B., Helm, M. L. & Bullock, R. M. Heterolytic cleavage of H2 by bifunctional manganese(i) complexes: impact of ligand dynamics, electrophilicity, and base positioning. Chem. Sci. 5, 4729–4741 (2014).
Yang, J. Y. et al. Two pathways for electrocatalytic oxidation of hydrogen by a nickel bis(diphosphine) complex with pendant amines in the second coordination sphere. J. Am. Chem. Soc. 135, 9700–9712 (2013).
Zhang, G., Scott, B. L. & Hanson, S. K. Mild and homogeneous cobalt-catalyzed hydrogenation of C=C, C=O, and C=N bonds. Angew. Chem. Int. Ed. 51, 12102–12106 (2012).
Kuriyama, W., Matsumoto, T., Ino, Y. & Ogata, O. WO2011048727A1 (2011).
Bertoli, M. et al. Osmium and ruthenium catalysts for dehydrogenation of alcohols. Organometallics 30, 3479–3482 (2011).
Geisser, R. W., Oetiker, J. D. & Schroeder, F. WO2015110515A1 (2015).
Nakayama, Y. & Ogata, O. WO2016031874A1 (2016).
Zell, T. & Langer, R. From ruthenium to iron and manganese — a mechanistic view on challenges and design principles of base-metal hydrogenation catalysts. ChemCatChem 10, 1930–1940 (2018).
Filonenko, G. A., van Putten, R., Hensen, E. J. M. & Pidko, E. A. Catalytic (de)hydrogenation promoted by non-precious metals — Co, Fe and Mn: recent advances in an emerging field. Chem. Soc. Rev. 47, 1459–1483 (2018).
Morris, R. H. Iron group hydrides in Noyori bifunctional catalysis. Chem. Rec. 16, 2644–2658 (2016).
Bullock, R. M. & Helm, M. L. Molecular electrocatalysts for oxidation of hydrogen using earth-abundant metals: shoving protons around with proton relays. Acc. Chem. Res. 48, 2017–2026 (2015).
Jing, Y., Chakraborty, S., Brennessel, W. W. & Jones, W. D. Additive-free cobalt-catalyzed hydrogenation of esters to alcohols. ACS Catal. 7, 3735–3740 (2017).
Bullock, R. M. Abundant metals give precious hydrogenation performance. Science 342, 1054–1055 (2013).
Hübner, S., de Vries, J. G. & Farina, V. Why does industry not use immobilized transition metal complexes as catalysts? Adv. Synth. Catal. 358, 3–25 (2016).
He, J. & Kappler, A. Recovery of precious metals from waste streams. Microb. Biotechnol. 10, 1194–1198 (2017).
Canda, L., Heput, T. & Ardelean, E. Methods for recovering precious metals from industrial waste. IOP Conf. Ser. Mater. Sci. Eng. 106, 012020 (2016).
Friedfeld, M. R., Zhong, H., Ruck, R. T., Shevlin, M. & Chirik, P. J. Cobalt-catalyzed asymmetric hydrogenation of enamides enabled by single-electron reduction. Science 360, 888–893 (2018).
Bullock, R. M. Reaction: earth-abundant metal catalysts for energy conversions. Chem 2, 444–446 (2017).
Korstanje, T. J., van der Vlugt, J. I., Elsevier, C. J. & de Bruin, B. Hydrogenation of carboxylic acids with a homogeneous cobalt catalyst. Science 350, 298–302 (2015).
Friedfeld, M. R. et al. Cobalt precursors for high-throughput discovery of base metal asymmetric alkene hydrogenation catalysts. Science 342, 1076–1080 (2013).
Jagadeesh, R. V. et al. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 342, 1073–1076 (2013).
Ford, E. S. et al. COPD Surveillance—United States, 1999–2011. Chest 144, 284–305 (2013).
Hughes, A. D. et al. Discovery of muscarinic acetylcholine receptor antagonist and beta 2 adrenoceptor agonist (MABA) dual pharmacology molecules. Bioorg. Med. Chem. Lett. 21, 1354–1358 (2011).
Osborne, R. et al. Efficient conversion of a nonselective norepinephrin reuptake inhibitor into a dual muscarinic antagonist−β2-agonist for the treatment of chronic obstructive pulmonary disease. J. Med. Chem. 54, 6998–7002 (2011).
Mammen, M. et al. US20040167167A1 (2004).
Komiyama, M., Itoh, T. & Takeyasu, T. Scalable ruthenium-catalyzed asymmetric synthesis of a key intermediate for the β2-Adrenergic receptor agonist. Org. Process Res. Dev. 19, 315–319 (2015).
Chung, J. Y. L. et al. Evolution of a manufacturing route to omarigliptin, a long-acting DPP-4 inhibitor for the treatment of type 2 diabetes. Org. Process Res. Dev. 19, 1760–1768 (2015).
Taylor, K. Drug in focus: levofloxacin. GenericsWeb https://web.archive.org/web/20140112065215/http://www.genericsweb.com/druginfocus/Levofloxacin (2010).
Hayakawa, I., Atarashi, S., Kimura, Y. & Kawakami, K. EP488227A2 (1992).
Johnson & Johnson. Investor relations business overview Q2 2010. investor.jnj http://files.shareholder.com/downloads/JNJ/6384228338x0x388829/8EC2A3A2-6953-45E2-AD82-656235F4D466/IR%20General%202Q10.pdf (2010).
Fujiwara, T. & Ebata, T. EP322815A2 (1989).
Brown Ripin, D. H. et al. Process improvements for the manufacture of tenofovir disoproxil fumarate at commercial scale. Org. Process Res. Dev. 14, 1194–1201 (2010).
Yoshikawa, N., Xu, F., Arredondo, J. D. & Itoh, T. A. Large-scale synthesis of potent glucokinase activator MK-0941 via selective O-arylation and O-alkylation. Org. Process Res. Dev. 15, 824–830 (2011).
Sumi, K. & Kumobayashi, H. in Organometallics in Process Chemistry (ed. Larsen, R.) 63–95 (Springer, Berlin Heidelberg, 2004).
Siebold, M. et al. Comparison of the production of lactic Acid by three different lactobacilli and its recovery by extraction and electrodialysis. Process Biochem. 30, 81–95 (1995).
Wee, Y.-J., Kim, J.-N. & Ryu, H.-W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 44, 163–172 (2006).
Ootsuka, T., Imamura, M., Ishii, A., Ueda, K. & Mimura, S. WO2014115801A1 (2014).
Otsuka, T., Ishii, A., Dub, P. A. & Ikariya, T. Practical selective hydrogenation of α-fluorinated esters with bifunctional pincer-type ruthenium(ii) catalysts leading to fluorinated alcohols or fluoral hemiacetals. J. Am. Chem. Soc. 135, 9600–9603 (2013).
Ishii, A., Ootsuka, T., Imamura, M., Nishimiya, T. & Kimura, K. WO2013018573A1 (2013).
Ishii, A., Ootsuka, T., Ishimaru, T. & Imamura, M. WO2012105431A1 (2012).
Dub, P. A., Scott, B. L. & Gordon, J. C. Why does alkylation of the N–H functionality within M/NH bifunctional Noyori-type catalysts lead to turnover? J. Am. Chem. Soc. 139, 1245–1260 (2017).
Li, H. et al. Rhenium and manganese complexes bearing amino-bis(phosphinite) ligands: synthesis, characterization, and catalytic activity in hydrogenation of ketones. Organometallics 37, 1271–1279 (2018).
Fu, S., Shao, Z., Wang, Y. & Liu, Q. Manganese-catalyzed upgrading of ethanol into 1-butanol. J. Am. Chem. Soc. 139, 11941–11948 (2017).
Kulkarni, N. V., Brennessel, W. W. & Jones, W. D. Catalytic upgrading of ethanol to n-butanol via manganese-mediated Guerbet reaction. ACS Catal. 8, 997–1002 (2018).
Fryzuk, M. D., MacNeil, P. A. & Rettig, S. J. Ancillary ligand involvement in the activation of dihydrogen by iridium(iii) complexes. Organometallics 4, 1145–1147 (1985).
Fryzuk, M. D., Montgomery, C. D. & Rettig, S. J. Synthesis and reactivity of ruthenium amide–phosphine complexes. Facile conversion of a ruthenium amide to a ruthenium amine via dihydrogen activation and orthometalation. X-Ray structure of RuCl(C6H4PPh2)[NH(SiMe2CH2PPh2)2]. Organometallics 10, 467–473 (1991).
Blum, Y., Czarkie, D., Rahamim, Y. & Shvo, Y. (Cyclopentadienone)ruthenium carbonyl complexes — a new class of homogeneous hydrogenation catalysts. Organometallics 4, 1459–1461 (1985).
Shvo, Y., Abed, M., Blum, Y. & Laine, R. M. Homogeneous catalytic cleavage of saturated carbon–nitrogen bonds. Isr. J. Chem. 27, 267–275 (1986).
Shvo, Y., Czarkie, D., Rahamim, Y. & Chodosh, D. F. A new group of ruthenium complexes: structure and catalysis. J. Am. Chem. Soc. 108, 7400–7402 (1986).
Noyori, R., Yamakawa, M. & Hashiguchi, S. Metal−ligand bifunctional catalysis: a nonclassical mechanism for asymmetric hydrogen transfer between alcohols and carbonyl compounds. J. Org. Chem. 66, 7931–7944 (2001).
Yamakawa, M., Yamada, I. & Noyori, R. CH/π attraction: the origin of enantioselectivity in transfer hydrogenation of aromatic carbonyl compounds catalyzed by chiral η6-arene-ruthenium(ii) complexes. Angew. Chem. Int. Ed. 40, 2818–2821 (2001).
Sandoval, C. A., Ohkuma, T., Muñiz, K. & Noyori, R. Mechanism of asymmetric hydrogenation of ketones catalyzed by BINAP/1,2-diamine–ruthenium(ii) complexes. J. Am. Chem. Soc. 125, 13490–13503 (2003).
Dub, P. A. & Ikariya, T. Quantum chemical calculations with the inclusion of nonspecific and specific solvation: asymmetric transfer hydrogenation with bifunctional ruthenium catalysts. J. Am. Chem. Soc. 135, 2604–2619 (2013).
Handgraaf, J.-W. & Meijer, E. J. Realistic modeling of ruthenium-catalyzed transfer hydrogenation. J. Am. Chem. Soc. 129, 3099–3103 (2007).
Pavlova, A. & Meijer, E. J. Understanding the role of water in aqueous ruthenium-catalyzed transfer hydrogenation of ketones. ChemPhysChem 13, 3492–3496 (2012).
Ohkuma, T. et al. trans-RuH(η1-BH4)(binap)(1,2-diamine): a catalyst for asymmetric hydrogenation of simple ketones under base-free conditions. J. Am. Chem. Soc. 124, 6508–6509 (2002).
Chen, C.-y. et al. Catalytic, enantioselective synthesis of taranabant, a novel, acyclic cannabinoid-1 receptor inverse agonist for the treatment of obesity. Org. Process Res. Dev. 11, 616–623 (2007).
Sandoval, C. A., Yamaguchi, Y., Ohkuma, T., Kato, K. & Noyori, R. Solution structures and behavior of trans-RuH(η1-BH4)(binap)(1,2-diamine) complexes. Magn. Reson. Chem. 44, 66–75 (2006).
Sandoval, C. A. et al. Mechanism of asymmetric hydrogenation of acetophenone catalyzed by chiral η6-arene–N-tosylethylenediamine–ruthenium(ii) complexes. Chem. Asian J. 1, 102–110 (2006).
Trincado, M. & Grützmacher, H. in Cooperative Catalysis (ed. Peters, R.) 67–110 (Wiley-VCH, Weinheim, 2015).
Grützmacher, H. Cooperating ligands in catalysis. Angew. Chem. Int. Ed. 47, 1814–1818 (2008).
Li, H. & Hall, M. B. Computational mechanistic studies on reactions of transition metal complexes with noninnocent pincer ligands: aromatization–dearomatization or not. ACS Catal. 5, 1895–1913 (2015).
Morris, R. H. Exploiting metal–ligand bifunctional reactions in the design of iron asymmetric hydrogenation catalysts. Acc. Chem. Res. 48, 1494–1502 (2015).
Hartmann, R. & Chen, P. Noyori’s hydrogenation catalyst needs a Lewis acid cocatalyst for high activity. Angew. Chem. Int. Ed. 40, 3581–3585 (2001).
Hartmann, R. & Chen, P. Numerical modeling of differential kinetics in the asymmetric hydrogenation of acetophenone by Nyori’s catalyst. Adv. Synth. Catal. 345, 1353–1359 (2003).
John, J. M., Takebayashi, S., Dabral, N., Miskolzie, M. & Bergens, S. H. Base-catalyzed bifunctional addition to amides and imides at low temperature. A new pathway for carbonyl hydrogenation. J. Am. Chem. Soc. 135, 8578–8584 (2013).
Dub, P. A., Henson, N. J., Martin, R. L. & Gordon, J. C. Unravelling the mechanism of the asymmetric hydrogenation of acetophenone by [RuX2(diphosphine)(1,2-diamine)] catalysts. J. Am. Chem. Soc. 136, 3505–3521 (2014).
Takebayashi, S., Dabral, N., Miskolzie, M. & Bergens, S. H. Experimental investigations of a partial Ru–O bond during the metal–ligand bifunctional addition in Noyori-type enantioselective ketone hydrogenation. J. Am. Chem. Soc. 133, 9666–9669 (2011).
Hamilton, R. J. & Bergens, S. H. Direct observations of the metal–ligand bifunctional addition step in an enantioselective ketone hydrogenation. J. Am. Chem. Soc. 130, 11979–11987 (2008).
Hamilton, R. J. & Bergens, S. H. An unexpected possible role of base in asymmetric catalytic hydrogenations of ketones. Synthesis and characterization of several key catalytic intermediates. J. Am. Chem. Soc. 128, 13700–13701 (2006).
Hamilton, R. J., Leong, C. G., Bigam, G., Miskolzie, M. & Bergens, S. H. A ruthenium−dihydrogen putative intermediate in ketone hydrogenation. J. Am. Chem. Soc. 127, 4152–4153 (2005).
Hasanayn, F. & Morris, R. H. Symmetry aspects of H2 splitting by five-coordinate d6 ruthenium amides, and calculations on acetophenone hydrogenation, ruthenium alkoxide formation, and subsequent hydrogenolysis in a model trans-Ru(H)2(diamine)(diphosphine) system. Inorg. Chem. 51, 10808–10818 (2012).
Dub, P. A. & Gordon, J. C. Metal–ligand bifunctional catalysis: the “accepted” mechanism, the issue of concertedness, and the function of the ligand in catalytic cycles Involving hydrogen atoms. ACS Catal. 7, 6635–6655 (2017).
Belkova, N. V., Epstein, L. M., Filippov, O. A. & Shubina, E. S. Hydrogen and dihydrogen bonds in the reactions of metal hydrides. Chem. Rev. 116, 8545–8587 (2016).
Karpfen, A. in Advances in Chemical Physics 469–510 (Wiley-VCH, Weinheim, 2003).
Armaroli, N. & Balzani, V. The hydrogen issue. ChemSusChem 4, 21–36 (2011).
Preuster, P., Papp, C. & Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 50, 74–85 (2017).
Trincado, M., Banerjee, D. & Grützmacher, H. Molecular catalysts for hydrogen production from alcohols. Energy Environ. Sci. 7, 2464–2503 (2014).
Goeppert, A., Czaun, M., Jones, J.-P., Surya Prakash, G. K. & Olah, G. A. Recycling of carbon dioxide to methanol and derived products — closing the loop. Chem. Soc. Rev. 43, 7995–8048 (2014).
Nielsen, M. et al. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 495, 85–89 (2013).
Rodríguez-Lugo, R. E. et al. A homogeneous transition metal complex for clean hydrogen production from methanol–water mixtures. Nat. Chem. 5, 342–347 (2013).
Kothandaraman, J., Goeppert, A., Czaun, M., Olah, G. A. & Surya Prakash, G. K. Conversion of CO2 from air into methanol using a polyamine and a homogeneous ruthenium catalyst. J. Am. Chem. Soc. 138, 778–781 (2016).
Alberico, E. et al. Unravelling the mechanism of basic aqueous methanol dehydrogenation catalyzed by Ru–PNP pincer complexes. J. Am. Chem. Soc. 138, 14890–14904 (2016).
Sinha, V., Govindarajan, N., de Bruin, B. & Meijer, E. J. How solvent affects C–H activation and hydrogen production pathways in homogeneous Ru-catalysed methanol dehydrogenation reactions. ACS Catal. 8, 6908–6913 (2018).
Wei, Z., de Aguirre, A., Junge, K., Beller, M. & Jiao, H. Exploring the mechanisms of aqueous methanol dehydrogenation catalyzed by defined PNP Mn and Re pincer complexes under base-free as well as strong base conditions. Catal. Sci. Technol. 8, 3649–3665 (2018).
Chandrasekhar, S. The principle of microscopic reversibility in organic chemistry — a critique. Res. Chem. Intermed. 17, 173–209 (1992).
Krupka, R. M., Kaplan, H. & Laidler, K. J. Kinetic consequences of the principle of microscopic reversibility. Trans. Faraday Soc. 62, 2754–2759 (1966).
Burwell, R. L. & Pearson, R. G. The principle of microscopic reversibility. J. Phys. Chem. 70, 300–302 (1966).
Gusev, D. G. Dehydrogenative coupling of ethanol and ester hydrogenation catalyzed by pincer-type YNP complexes. ACS Catal. 6, 6967–6981 (2016).
Vicent, C. & Gusev, D. G. ESI-MS insights into acceptorless dehydrogenative coupling of alcohols. ACS Catal. 6, 3301–3309 (2016).
Spasyuk, D. & Gusev, D. G. Acceptorless dehydrogenative coupling of ethanol and hydrogenation of esters and imines. Organometallics 31, 5239–5242 (2012).
Zweifel, T., Naubron, J. V. & Grützmacher, H. Catalyzed dehydrogenative coupling of primary alcohols with water, methanol, or amines. Angew. Chem. Int. Ed. 48, 559–563 (2009).
van Putten, R. et al. Non-pincer-type manganese complexes as efficient catalysts for the hydrogenation of esters. Angew. Chem. Int. Ed. 56, 7531–7534 (2017).
Chen, X., Jing, Y. & Yang, X. Unexpected direct hydride transfer mechanism for the hydrogenation of ethyl acetate to ethanol catalyzed by SNS pincer ruthenium complexes. Chem. Eur. J. 22, 1950–1957 (2016).
Zhang, L. et al. Acceptorless dehydrogenative coupling of alcohols catalysed by ruthenium PNP complexes: influence of catalyst structure and of hydrogen mass transfer. J. Catal. 340, 331–343 (2016).
Qu, S. et al. Computational mechanistic study of Fe-catalyzed hydrogenation of esters to alcohols: improving catalysis by accelerating precatalyst activation with a Lewis base. ACS Catal. 4, 4377–4388 (2014).
Chen, T. et al. Hydrogenation of esters catalyzed by ruthenium PN3-pincer complexes containing an aminophosphine arm. Organometallics 33, 4152–4155 (2014).
Nguyen, D. H. et al. Deeper mechanistic insight into Ru pincer-mediated acceptorless dehydrogenative coupling of alcohols: exchanges, intermediates, and deactivation species. ACS Catal. 8, 4719–4734 (2018).
Hasanayn, F. & Baroudi, A. Direct H/OR and OR/ORʹ metathesis pathways in ester hydrogenation and transesterification by Milstein’s catalyst. Organometallics 32, 2493–2496 (2013).
Ito, M. et al. Catalytic hydrogenation of carboxamides and esters by well-defined Cp*Ru complexes bearing a protic amine ligand. J. Am. Chem. Soc. 133, 4240–4242 (2011).
Hayes, J. M. et al. Ketone hydrogenation with iridium complexes with “non N–H” ligands: the key role of the strong base. ACS Catal. 5, 4368–4376 (2015).
Gu, G. et al. Iridium/f-ampha-catalyzed asymmetric hydrogenation of aromatic α-keto esters. Org. Chem. Front. 5, 1209–1212 (2018).
Wang, Z. et al. Cooperative interplay between a flexible PNN-Ru(ii) complex and a NaBH4 additive in the efficient catalytic hydrogenation of esters. Catal. Sci. Technol. 7, 1297–1304 (2017).
Gu, G. et al. Enantioselective iridium-catalyzed hydrogenation of α-keto amides to α-hydroxy amides. Org. Lett. 19, 5920–5923 (2017).
Liu, C., van Putten, R., Kulyaev, P. O., Filonenko, G. A. & Pidko, E. A. Computational insights into the catalytic role of the base promoters in ester hydrogenation with homogeneous non-pincer-based Mn-P,N catalyst. J. Catal. 363, 136–143 (2018).
Moore, C. M., Bark, B. & Szymczak, N. K. Simple ligand modifications with pendent OH groups dramatically impact the activity and selectivity of ruthenium catalysts for transfer hydrogenation: the importance of alkali metals. ACS Catal. 6, 1981–1990 (2016).
Liang, Z., Yang, T., Gu, G., Dang, L. & Zhang, X. Scope and mechanism on iridium-f-amphamide catalyzed asymmetric hydrogenation of ketones. Chin. J. Chem. 36, 851–856 (2018).
Dub, P. A. & Ikariya, T. Catalytic reductive transformations of carboxylic and carbonic acid derivatives using molecular hydrogen. ACS Catal. 2, 1718–1741 (2012).
Ojeda-Porras, A. & Gamba-Sánchez, D. Recent developments in amide synthesis using nonactivated starting materials. J. Org. Chem. 81, 11548–11555 (2016).
Gunanathan, C., Ben-David, Y. & Milstein, D. Direct synthesis of amides from alcohols and amines with liberation of H2. Science 317, 790–792 (2007).
Zweifel, T., Naubron, J.-V. & Grützmacher, H. Catalyzed dehydrogenative coupling of primary alcohols with water, methanol, or amines. Angew. Chem., Int. Ed. 48, 559–563 (2009).
Oldenhuis, N. J., Dong, V. M. & Guan, Z. Catalytic acceptorless dehydrogenations: Ru-MACHO catalyzed construction of amides and imines. Tetrahedron 70, 4213–4218 (2014).
Zhang, G., Yin, Z. & Zheng, S. Cobalt-catalyzed N-alkylation of amines with alcohols. Org. Lett. 18, 300–303 (2016).
Landge, S. M., Borkin, D. A. & Török, B. Microwave-assisted preparation of trifluoroacetaldehyde (fluoral): isolation and applications. Tetrahedron Lett. 48, 6372–6376 (2007).
Kwiecień, A. & Ciunik, Z. Stable hemiaminals: 2-aminopyrimidine derivatives. Molecules 20, 14365–14376 (2015).
Mispelaere, C. & Roques, N. Hemiaminals of trifluoroacetaldehyde, as trifluoromethylating agents. Tetrahedron Lett. 40, 6411–6414 (1999).
Folléas, B., Marek, I., Normant, J.-F. & Saint-Jalmes, L. Fluoroform: an efficient precursor for the trifluoromethylation of aldehydes Tetrahedron 56, 275–283 (2000).
Ogata, O., Nara, H. & Nakayama, Y. Preparation of ruthenium complexes having bis(phosphinoalkyl)amine and N-heterocyclic carbene ligands, organic reaction catalysts containing them, and their use. WO2015163440A1 (2015).
Artús Suàrez, L. et al. The Key Role of the Hemiaminal intermediate in the iron-catalyzed deaminative hydrogenation of amides. ACS Catal. 8, 8751–8762 (2018).
Gusev, D. G. Rethinking the dehydrogenative amide synthesis. ACS Catal. 7, 6656–6662 (2017).
Love, B. E., Boston, T. S., Nguyen, B. T. & Rorer, J. R. A comparison of imine forming methodologies. Org. Prep. Proced. Int. 31, 399–405 (1999).
Mascavage, L. M., Sonnet, P. E. & Dalton, D. R. On the surface-catalyzed reaction between the gases 2,2-dimethylpropanal and methanamine. Formation of active-site imines. J. Org. Chem. 71, 3435–3443 (2006).
Eisenstein, O. & Crabtree, R. H. Outer sphere hydrogenation catalysis. New J. Chem. 37, 21–27 (2013).
Mills, M. R., Barnes, C. L. & Bernskoetter, W. H. Influences of bifunctional PNP-pincer ligands on low valent cobalt complexes relevant to CO2 hydrogenation. Inorg. Chem. 57, 1590–1597 (2018).
Puylaert, P. et al. Selective hydrogenation of α, β-unsaturated aldehydes and ketones by air-stable ruthenium NNS complexes. Chem. Eur. J. 23, 8473–8481 (2017).
Gorgas, N., Stöger, B., Veiros, L. F. & Kirchner, K. Highly efficient and selective hydrogenation of aldehydes: a well-defined Fe(ii) catalyst exhibits noble-metal activity. ACS Catal. 6, 2664–2672 (2016).
Mellone, I. et al. Selective formic acid dehydrogenation catalyzed by Fe-PNP pincer complexes based on the 2,6-diaminopyridine scaffold. Organometallics 35, 3344–3349 (2016).
Zhang, L., Han, Z., Zhao, X., Wang, Z. & Ding, K. Highly efficient ruthenium-catalyzed N-formylation of amines with H2 and CO2. Angew. Chem. Int. Ed. 54, 6186–6189 (2015).
Zhang, Y. et al. Iron catalyzed CO2 hydrogenation to formate enhanced by Lewis acid co-catalysts. Chem. Sci. 6, 4291–4299 (2015).
Curley, J. B., Smith, N. E., Bernskoetter, W. H., Hazari, N. & Mercado, B. Q. Catalytic formic acid dehydrogenation and CO2 hydrogenation using iron PNRP pincer complexes with isonitrile ligands. Organometallics https://doi.org/10.1021/acs.organomet.8b00534 (2018).
Lundgren, R. J. & Stradiotto, M. Rapid ketone transfer hydrogenation by employing simple, in situ prepared iridium(i) precatalysts supported by “non-N–H” P,N ligands. Chem. Eur. J. 14, 10388–10395 (2008).
Lundgren, R. J., Rankin, M. A., McDonald, R., Schatte, G. & Stradiotto, M. A formally zwitterionic ruthenium catalyst precursor for the transfer hydrogenation of ketones that does not feature an ancillary ligand N–H functionality. Angew. Chem. 119, 4816–4819 (2007).
Sinopalnikova, I. S. et al. Ruthenium p-cymene iminophosphonamide complexes: activation under basic conditions and transfer hydrogenation catalysis. Eur. J. Inorg. Chem. 2018, 2285–2299 (2018).
Schilter, D., Camara, J. M., Huynh, M. T., Hammes-Schiffer, S. & Rauchfuss, T. B. Hydrogenase enzymes and their synthetic models: the role of metal hydrides. Chem. Rev. 116, 8693–8749 (2016).
Ogata, H., Lubitz, W. & Higuchi, Y. Structure and function of [NiFe] hydrogenases. J. Biochem. 160, 251–258 (2016).
Peters, J. W. et al. [FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochim. Biophys. Acta, Mol. Cell Res. 1853, 1350–1369 (2015).
Lubitz, W., Ogata, H., Rüdiger, O. & Reijerse, E. Hydrogenases. Chem. Rev. 114, 4081–4148 (2014).
Mulder, D. W. et al. Insights into [FeFe]-hydrogenase structure, mechanism, and maturation. Structure 19, 1038–1052 (2011).
Madden, C. et al. Catalytic turnover of [FeFe]-hydrogenase based on single-molecule imaging. J. Am. Chem. Soc. 134, 1577–1582 (2012).
Pandey, A. S., Harris, T. V., Giles, L. J., Peters, J. W. & Szilagyi, R. K. Dithiomethylether as a ligand in the hydrogenase H-cluster. J. Am. Chem. Soc. 130, 4533–4540 (2008).
Peters, J. W., Lanzilotta, W. N., Lemon, B. J. & Seefeldt, L. C. X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282, 1853–1858 (1998).
Nicolet, Y., Piras, C., Legrand, P., Hatchikian, C. E. & Fontecilla-Camps, J. C. Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 7, 13–23 (1999).
Mulder, D. W. et al. Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG. Nature 465, 248–251 (2010).
Lemon, B. J. & Peters, J. W. Binding of exogenously added carbon monoxide at the active site of the iron-only hydrogenase (CpI) from Clostridium pasteurianum. Biochem 38, 12969–12973 (1999).
Silakov, A., Wenk, B., Reijerse, E. & Lubitz, W. 14N HYSCORE investigation of the H-cluster of [FeFe] hydrogenase: evidence for a nitrogen in the dithiol bridge. Phys. Chem. Chem. Phys. 11, 6592–6599 (2009).
Lubitz, W., Reijerse, E. & van Gastel, M. [NiFe] and [FeFe] hydrogenases studied by advanced magnetic resonance techniques. Chem. Rev. 107, 4331–4365 (2007).
Carr, S. B. et al. Hydrogen activation by [NiFe]-hydrogenases. Biochem. Soc. Trans. 44, 863–868 (2016).
Kalz, K. F., Brinkmeier, A., Dechert, S., Mata, R. A. & Meyer, F. Functional model for the [Fe] hydrogenase inspired by the frustrated Lewis pair concept. J. Am. Chem. Soc. 136, 16626–16634 (2014).
Pelmenschikov, V. et al. Reaction coordinate leading to H2 production in [FeFe]-hydrogenase Identified by nuclear resonance vibrational spectroscopy and density functional theory. J. Am. Chem. Soc. 139, 16894–16902 (2017).
Belkova, N. V., Filippov, O. A. & Shubina, E. S. Z−H bond activation in (di)hydrogen bonding as a way to proton/hydride transfer and H2 evolution. Chem. Eur. J. 24, 1464–1470 (2018).
Belkova, N. V., Shubina, E. S. & Epstein, L. M. Diverse world of unconventional hydrogen bonds. Acc. Chem. Res. 38, 624–631 (2005).
Sadeghi, R. R. & Cheng, H.-P. The dynamics of proton transfer in a water chain. J. Chem. Phys. 111, 2086–2094 (1999).
Hong, G., Cornish, A. J., Hegg, E. L. & Pachter, R. On understanding proton transfer to the biocatalytic [Fe–Fe]H sub-cluster in [Fe–Fe]H2ases: QM/MM MD simulations. Biochim. Biophys. Acta, Bionenerg. 1807, 510–517 (2011).
Long, H., King, P. W. & Chang, C. H. Proton transport in Clostridium pasteurianum [FeFe] hydrogenase I: a computational study. J. Phys. Chem. B 118, 890–900 (2014).
Dub, P. A. et al. Hydrogen bonding to carbonyl hydride complex Cp*Mo(PMe3)2(CO)H and its role in proton transfer. Dalton Trans. 39, 2008–2015 (2010).
Ayllon, J. A., Sayers, S. F., Sabo-Etienne, S., Donnadieu, B. & Chaudret, B. Proton transfer in aminocyclopentadienyl ruthenium hydride complexes. Organometallics 18, 3981–3990 (1999).
Acknowledgements
Various aspects of the work that formed the foundation of this Review were graciously supported by the Laboratory Directed Research and Development (LDRD) programme at Los Alamos National Laboratory.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dub, P.A., Gordon, J.C. The role of the metal-bound N–H functionality in Noyori-type molecular catalysts. Nat Rev Chem 2, 396–408 (2018). https://doi.org/10.1038/s41570-018-0049-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0049-z
This article is cited by
-
A 13-million turnover-number anionic Ir-catalyst for a selective industrial route to chiral nicotine
Nature Communications (2023)
-
Chiral, air stable, and reliable Pd(0) precatalysts applicable to asymmetric allylic alkylation chemistry
Nature Communications (2023)
-
Isolating intermediates
Nature Chemistry (2022)
-
Structure, reactivity and catalytic properties of manganese-hydride amidate complexes
Nature Chemistry (2022)
-
Computational Studies on the Mechanisms for Deaminative Amide Hydrogenation by Homogeneous Bifunctional Catalysts
Topics in Catalysis (2022)