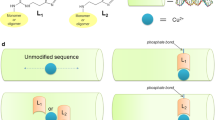
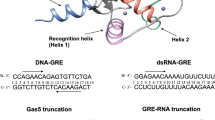
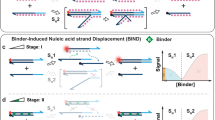
Abstract
Considerable recent effort has been devoted to the design and selection of sequence-specific DNA binding proteins based on tandem arrays of Cys2His2 zinc finger domains. While the DNA binding properties of these designed proteins have been studied extensively, the structural basis for site-specific binding has not been examined experimentally. Here we report the crystal structure of a complex between a protein comprised of three consensus-sequence-based zinc finger domains and an oligonucleotide corresponding to a favourable DNA binding site. This structure reveals relatively simple modular interactions and structural adaptations that compensate for differences in contact residue side-chain lengths.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Miller, J., McLachlan, A.D. & Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 4, 1609–1614 (1985).
Rhodes, D. & Klug, A. An underlying repeat in sometranscriptional control sequences corresponding to half a double helical turn of DNA. Cell 46, 123–132 (1986).
Nardelli, J., Gibson, T.J., Vesque, C. & Charnay, P. Base sequence discrimination by zinc-finger DNA-binding domains. Nature 349, 175 (1991).
Pavletich, N.P. & Pabo, C.O. Zinc finger -DNA recognition: Crystal structure of a Zif268-DNA complex at 2. 1 Å resolution. Science 252, 809 (1991).
Lee, M.S., Gippert, G.P., Soman, K.V., Case, D.A. & Wright, P.E. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science 245, 635–637 (1988).
Nekludova, L. & Pabo, C.O. Distinctive DNA conformation with enlarged major groove is found in Zn-finger-DNA and other protein-DNA complexes. Proc. Natl. Acad. Sci. USA 91, 6948–6952 (1994).
Pavletich, N.P. & Pabo, C.O. Crystal structure of a five-finger GLI-DNA complex: New perspectives on zinc fingers. Science 261, 1701–1707 (1993).
Fairall, L., Schwabe, J.W.R., Chapman, L., Finch, J.T. & Rhodes, D. The crystal structure of a two zinc-finger peptide reveals an extension to the rules for zinc-finger/DNA recognition. Nature 366, 483–487 (1993).
Rebar, E.J. & Pabo, C.O. Zinc finger phage: affinity selection of fingers with new DNA-binding specificities. Science 263, 671–673 (1994).
Choo, Y. & Klug, A. Toward a code for the interactions of zinc fingers with DNA: selection of randomized fingers displayed on phage. Proc. Natl. Acad. Sci. USA 91, 11163–11167 (1994).
Jamieson, A.C., Kim, S.-H. & Wells, J.A. In vitro selection of zinc fingers with altered DNA-binding specificity. Biochemistry 33, 5689–5695 (1994).
Wu, H., Yang, W.-P. & Barbas, C.F. III Building zinc fingers by selection: toward a therapeutic application. Proc. Natl. Acad. Sci, USA 92, 344–348 (1995).
Choo, Y., Sanchez-Garcia, I. & Klug, A. In vivo repression by a site-specific DNA-binding protein designed against an oncogenic sequence. Nature 372, 642–645 (1994).
Nardelli, J., Gibson, T. & Charnay, P. Zinc finger-DNA recognition analysis of base specificity by site-directed mutagenesis. Nucl. Acid. Res. 20, 4137–4144 (1992).
Thukral, S.K., Morrison, M.L. & Young, E.T. Mutations in the zinc fingers of ADR1 that change the specificity of DNA binding and transactivation. Mol. Cell. Biol. 12, 2784–2792 (1992).
Desjarlais, J.R. & Berg, J.M. Redesigning the DNA-binding specificity of a zinc finger protein: a data base guided approach. Proteins Struct. Funct Genet. 12, 101–104 (1992); Desjarlais, J.R. & Berg, J.M. Erratum: Redesigning the DNA-binding specificity of a zinc finger protein: a data base guided approach. Proteins Struct. Funct. Genet 13, 272 (1992).
Desjarlais, J.R. & Berg, J.M. Toward rules relating zinc finger protein sequences and DNA binding site preferences. Proc. Natl. Acad. Sci. USA 89, 7345–7349 (1992).
Krizek, B.A., Amann, B.T., Kilfoil, V.J., Merkle, D.L. & Berg, J.M. A consensus zinc finger peptide: design, high affinity metal binding, pH dependent structure, and a His to Cys sequence variant. J. Am. Chem. Soc. 113, 4518–4523 (1991).
Desjarlais, J.R. & Berg, J.M. Using a zinc finger consensus framework and specificity rules to design specific DNA binding proteins. Proc. Natl. Acad. Sci. USA 90, 2256–2260 (1993).
Desjarlais, J.R. & Berg, J.M. Length-encoded multiplex binding site determination: application to zinc finger proteins. Proc. Natl. Acad. Sci. USA 91, 11099–11103 (1994).
Shi, Y. & Berg, J.M. A direct comparison of the properties of natural and designed zinc finger proteins. Chem. Biol. 2, 83–89 (1995).
Kim, C.A. & Berg, J.M. Ser is position 2 in the DNA recognition helix of a Cys2His2 zinc finger peptide is not, in general, responsible for base recognition. J. Mol. Biol. 252, 1–5 (1995).
Pabo, C.O. & Sauer, R.T. Transcription factors: structural families and principles of DNA recognition. Ann. Rev. Biochem. 61, 1053–1095 (1992).
Shi, Y. & Berg, J.M. DNA unwinding induced by zinc finger protein binding. Biochemistry 35, 3845–3848 (1996).
Tainer, J.A., Getzoff, E.D., Beem, K.M., Richardson, J.S. & Richardson, D.C. Determination and analysis of the 2 Å structure of copper-zinc superoxide dismutase. J. Mol. Biol. 160, 181–217 (1982).
Coughlan, P.K. & Lippard, S.J. Magnetic, ESR, electrochemical, and potentiometric titration studies of the imidazolate-bridged dicopper(II) ion in a binucleating macrocycle. Inorganic Chem. 23, 1446–1451 (1984).
Nakaseko, Y., Neuhaus, D., Klug, A. & Rhodes, D. Adjacent zinc-finger motifs in multiple zinc-finger proteins from SWI5 form structurally independent, flexibly linked domains. J. Mol. Biol. 228, 619–636 (1992).
Brünger, A.T. X-PLOR Version 3.1: A System for X-ray Crystallography and NMR (Yale University Press, New Haven, CT, 1992).
Lavery, R. & Sklenar, H. The definition of generalized helicoidal parameters and an axis of curvature for irregular nucleic acids. J. Biomolec. Struct. Dyn. 6, 63–91 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, C., Berg, J. A 2.2 Å resolution crystal structure of a designed zinc finger protein bound to DNA. Nat Struct Mol Biol 3, 940–945 (1996). https://doi.org/10.1038/nsb1196-940
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1196-940
This article is cited by
-
Zinc binding of a Cys2His2-type zinc finger protein is enhanced by the interaction with DNA
JBIC Journal of Biological Inorganic Chemistry (2023)
-
Modulating Mitochondrial DNA Heteroplasmy with Mitochondrially Targeted Endonucleases
Annals of Biomedical Engineering (2022)
-
Design of a colicin E7 based chimeric zinc-finger nuclease
Journal of Computer-Aided Molecular Design (2014)
-
Structure and dynamics of a primordial catalytic fold generated by in vitro evolution
Nature Chemical Biology (2013)
-
Inhibition of Binding of Tomato Yellow Leaf Curl Virus Rep to its Replication Origin by Artificial Zinc-Finger Protein
Molecular Biotechnology (2013)