Key Points
-
IgA nephropathy is defined by the presence of IgA-dominant or co-dominant glomerular deposits
-
Glomerular changes under light microscopy are diverse, ranging from minimal abnormality to crescentic glomerulonephritis, or an appearance resembling primary focal segmental glomerulosclerosis
-
Mesangial hypercellularity, segmental glomerulosclerosis and tubular atrophy/interstitial fibrosis are of prognostic value and predict renal outcomes independent of clinical variables
-
Evidence from retrospective clinicopathological studies indicates that endocapillary hypercellularity and cellular crescents are responsive to steroid and/or immunosuppressive therapy
-
Prospective data from randomized control trials are required to determine how histopathology should be used to guide therapy
Abstract
IgA nephropathy is defined by the presence of IgA-dominant or co-dominant immune deposits within glomeruli. Biopsy specimens meeting these diagnostic criteria have a range of histological changes that are reflected in the variable clinical course of IgA nephropathy. The impact of histology on outcomes in IgA nephropathy has been clarified in a number of large retrospective clinicopathological studies. These studies have consistently demonstrated that the stage of disease at presentation, as indicated by the extent of interstitial fibrosis and tubular atrophy in the biopsy, is the strongest histological predictor of renal survival. The effect of active proliferative lesions on the disease course is less clear cut, owing in part to considerable treatment bias in most published retrospective studies. There is evidence that endocapillary hypercellularity and cellular crescents are responsive to immunosuppressive therapy, but this observation requires confirmation in prospective randomized controlled trials. Future challenges include improving the reproducibility of histological scoring, particularly for the presence and extent of endocapillary lesions, and to improve prognostic modelling by combining histological data with clinical variables and biomarker data.
Similar content being viewed by others
Introduction
IgA nephropathy is defined by immunohistology, specifically the presence of IgA-dominant or co-dominant immune deposits in the glomeruli. Patients with lupus nephritis, IgA-dominant postinfectious glomerulonephritis and a pure membranous distribution of IgA deposits (a rare phenomenon) are excluded from this definition. IgA nephropathy is the most common glomerular disease worldwide, with the notable exception of sub-Saharan Africa, where it accounts for only 1% of black patients with primary glomerular disease.1 The prevalence of this disease is particularly high in South East Asia, where IgA nephropathy accounts for 30–50% of renal biopsy diagnoses.2
The spectrum of histological changes that might be seen in a renal biopsy meeting the definition of IgA nephropathy is wide. These changes, visible under light microscopy, range from those that are near normal to severe proliferative glomerulonephritis with cellular crescents, or a morphology that resembles primary focal segmental glomerulosclerosis (FSGS). Histological diversity is reflected in the variable clinical presentations of IgA nephropathy, which range from asymptomatic microscopic haematuria to rapidly progressive glomerulonephritis or nephrotic syndrome. In the presence of such clinical and histological diversity, whether or not IgA nephropathy is a single disease has been questioned. Certainly, geographical and demographic variations in the clinical presentation of IgA nephropathy are unexplained. In North America and Europe, IgA nephropathy is strikingly predominant in males, with a male to female ratio of 2–3:1,3 whereas in South East Asia the male to female ratio in disease incidence is equal.4 Variations exist in the frequency of the different histological patterns that might reflect differences in biopsy policy between centres, but might also result from true geographical variation in the disease.
The pathogenesis of IgA nephropathy is incompletely understood and is beyond the scope of this Review, but is discussed in detail elsewhere.5 Evidence for the presence of abnormally glycosylated polymeric IgA1 in serum, and in glomerular deposits in patients with IgA nephropathy, probably reflects abnormal production of mucosal-type IgA1 in the bone marrow, and is associated with autoantibodies (IgG or IgA) against undergalactosylated IgA1.6 These immunological abnormalities are not necessarily associated with glomerular deposition of IgA and are frequently present in relatives of patients with IgA nephropathy in the absence of evidence of renal disease. The factors that determine the deposition and location of IgA in glomeruli are unknown, but some evidence suggests that immune complexes of aberrantly glycosylated IgA1 show greater binding to mesangial cells than does uncomplexed IgA.7 Glomerular IgA deposits are typically diffuse; however, in early disease they might be present in only a small proportion of glomeruli as focal deposits. This disease stage is rarely seen in primary IgA nephropathy in native kidneys, but is encountered in early post-transplant recurrence.8 The basis for the histological and clinical diversity in this disease is poorly understood; autopsy findings demonstrate a high prevalence of mesangial IgA deposition in some populations even in the absence of the symptoms of renal disease.9 IgA deposition alone is clearly insufficient to lead to glomerular injury and the clinical manifestations of IgA nephropathy.
In this Review, I focus on the spectrum of pathological changes in IgA nephropathy, discuss the various systems of classification of histological changes and the relationship between pathology and clinical outcomes. I start with a detailed description of the immunohistology of IgA nephropathy and discuss the potential roles for IgG and complement factors in mediating glomerular damage. The histological lesions seen in IgA nephropathy are defined and illustrated; accurate recognition of the various lesions is essential for reproducible classification of the disease. The different schemas for classification of IgA nephropathy are compared with particular focus on the Oxford Classification. This Review immediately precedes the first Oxford Conference on IgA nephropathy to be held in June 2014.
Immunohistology of IgA nephropathy
The presence of IgA deposits within glomeruli can be demonstrated by immunohistochemisty and fluorescence microscopy of frozen tissue sections or by immunoperoxidase staining of paraffin embedded tissue sections (Figure 1). Deposits comprise polymeric IgA, with predominantly the IgA1 subclass.10 In native IgA nephropathy, there is diffuse glomerular positivity for IgA at the time of clinical presentation, but after therapy this becomes focal or even negative. In a study of 35 patients with IgA nephropathy treated by tonsillectomy, pulse methylprednisolone and a 1-year course of prednisolone, all patients had initial biopsies with >80% of glomeruli positive for IgA.11 By contrast, IgA immunoreactivity was completely negative in a repeat biopsy at a mean 77 months later in 23% of patients.11 This study highlights that therapeutic intervention not only suppresses inflammation and proliferation in glomeruli, but modulates the process that underlies renal IgA deposition.
a | Immunofluorescence microscopy and b | immunoperoxidase staining demonstrate mesangial IgA deposits. c | Electron microscopy shows mesangial (black arrowheads) and subendothelial (black arrow) electron-dense deposits. Periodic acid–Schiff-stained sections with different patterns of glomerular involvement: d | mesangial hypercellularity only, e | segmental endocapillary hypercellularity associated with a cellular crescent and f | segmental sclerosis.
IgA deposits are most commonly detected only in the mesangium but additional capillary wall deposits are found in up to one-third of patients (Figure 1). The mesangial IgA deposits, viewed by electron microscopy, are unstructured and typically situated immediately beneath the basement membrane as it crosses the mesangium (the 'paramesangial area'). Capillary wall IgA deposits are in a subendothelial location; subepithelial deposits are rare.
Capillary wall IgA deposits are associated with greater histological activity, as indicated by increased mesangial and endocapillary hypercellularity.12 In some studies capillary wall IgA deposits are associated with an increased frequency of crescents and more advanced chronic renal damage.13,14 The association of capillary wall IgA with increased proliferative activity and advanced fibrosis is reflected in the clinical outcome of these patients. In several studies, the presence of capillary wall deposits has signified a worse renal prognosis.15 The Oxford classification of IgA nephropathy identifies four histological features that are independent predictors of clinical outcome but does not include immunohistology.16,17 Reanalysis of biopsy reports in the Oxford classification patient cohort indicated that the location of glomerular IgA deposits is not an independent predictor of outcome when the histological lesions that form the basis of this schema are included in a multivariate analysis.12
Immunofluorescent microscopy might reveal glomerular IgG in addition to IgA deposits. The frequency of IgG positivity varies greatly between different cohorts, ranging from 15–85% within published series,12,17,20 which might reflect differences in patient age, stage of disease at the time of biopsy or true geographical variation in the immunohistology of IgA nephropathy. In the Oxford classification cohort, 25% of biopsy specimens had more than trace levels of IgG.12 A weak association between glomerular IgG deposits and serum levels of galactose-deficient IgA1 was demonstrated in another study.21 The presence of IgG has been associated with more aggressive clinical disease and poorer renal outcomes than the presence of IgA deposits alone.15,22 In a Japanese cohort, patients with glomerular IgG deposits had greater proteinuria at presentation and a lower rate of remission of urinary abnormalities after treatment than patients with IgA deposits alone.23
Complement component 3 (C3) is present in >90% of biopsy samples, but typically C1q (the first subcomponent of the C1 complex of the classical pathway of complement activation) is absent. The amount of mesangial C3 deposition might be of prognostic value; strong glomerular positivity for C3 on immunofluorescence and low levels of serum C3 were associated with loss of renal function in a report on IgA nephropathy from Korea.24 Glomerular deposits of complement degradation product C4d are observed in one-third to one-half of IgA nephropathy biopsy samples; in two studies, the presence of these deposits correlated with increased disease activity25 and subsequent development of end-stage renal disease (ESRD).26
Histological lesions
Glomerular histology in IgA nephropathy is highly variable and it is essential to precisely identify the morphology to achieve a reproducible classification. The challenge for pathologists is to provide accurate descriptions and definitions to minimize interobserver variation in reporting renal histology. Reproducibility is most easily achieved with a small group of pathologists working together, as in the Oxford classification study.17 Lesions that seem highly reproducible in these studies, however, might not be so in routine diagnostic practice, when comparing pathologists' findings from different laboratories or countries. This disparity is evident in the application of the Banff classification of transplant pathology, with which there was poor agreement between pathologists in the scoring of many lesions in a European slide circulation,27 and in the VALIGA study, a pan-European validation study of the Oxford classification of IgA nephropathy.28 Renal biopsies from 1,147 patients in 55 centres around Europe were scored in the VALIGA study, initially by the local pathologists, and reviewed centrally in Oxford. Agreement between local and review pathologists in the scoring of lesions was good for tubular atrophy/interstitial fibrosis and segmental sclerosis, moderate for mesangial hypercellularity and poor for endocapillary hypercellularity (I. S. D. Roberts, unpublished data). In the following sections, I illustrate the various lesions and highlight difficulties in categorisation.
Minimal abnormality
Glomeruli might appear near normal early in disease with little proliferative activity. To define 'normal by light microscopy' in IgA nephropathy is more challenging than for conditions such as pauci-immune vasculitis, because the presence of mesangial IgA deposits is associated with subtle histological changes, even in the absence of overt cellular proliferation or sclerosis. The IgA deposits, particularly if extensive, can be seen in periodic acid–Schiff (PAS)-stained or silver-stained tissue sections. In the Oxford classification study, the intraclass correlation coefficient (equivalent to the kappa statistic in multiple rater analyses) for the number of normal glomeruli was low (0.27), reflecting the difficulty in defining 'normality'.17 'Minimal abnormality' might be a preferable term to 'normal' and could be defined by the absence of hypercellularity, sclerosis or hyalinosis. The proportion of biopsies that show minimal abnormality ranges from 8–55%,29,30,31 reflecting differences in biopsy practice. For example, centres that carry out renal biopsies in patients with isolated haematuria will probably have a higher proportion of biopsies that show minimal abnormality than those centres that do not carry out biopsies in the absence of proteinuria.
Mesangial hypercellularity
The majority of biopsies from patients with IgA nephropathy demonstrate focal or diffuse mesangial hypercellularity that might vary from mild to severe (Figure 1). Mesangial hypercellularity is often accompanied by an increase in mesangial matrix that might be out of proportion to the increase in cellularity. To improve interobserver reproducibility, the Oxford classification working group produced definitions of the various histological lesions in IgA nephropathy. Mesangial hypercellularity is defined as greater than three mesangial cell nuclei per mesangial area in a 3 μm thick paraffin-embedded section. Cellularity should be assessed in PAS-stained tissue sections and exclude the central and perihilar region of the glomerulus. Mesangial cell nuclei are defined as those that are surrounded by PAS-positive extracellular matrix, in contrast to endothelial cell nuclei that project into the lumina of capillaries.
Endocapillary hypercellularity
Endocapillary hypercellularity—defined as an increased number of cells within glomerular capillaries, causing narrowing of the lumina (Figure 1)—is observed in around one-third of biopsy samples and is typically focal. The hypercellularity might reflect proliferation, inflammatory cell infiltration or endothelial cell swelling. Endocapillary lesions are more common in children and associated with active disease and high levels of proteinuria.15 The distinction of global endocapillary hypercellularity from segmental endocapillary lesions (including those associated with necrosis and crescents) is not made in most series. In the Oxford classification study, segmental and global endocapillary lesions were merged for the purpose of analysis to improve interobserver reproducibility. However, segmental and global lesions might reflect different mechanisms of injury, as suggested by the association of capillary wall IgA deposits with endocapillary hypercellularity but not with necrotizing or crescentic lesions.11 Whether or not this distinction has prognostic or therapeutic implications is currently unknown.
Interobserver reproducibility in the recognition of endocapillary hypercellularity is particularly challenging in the presence of sclerosis; cellular segmental sclerosing lesions are a frequent finding in IgA nephropathy, which is not surprising as the inflammatory process might evolve from an early proliferative phase to fibrosis. When scoring such lesions in tissue sections, only cells within the lumina of capillaries, and not those surrounded by extracellular matrix, should be used to define endocapillary hypercellularity. In IgA nephropathy, as in other proliferative glomerulonephritides, many of the glomerular cells are leukocytes.32 The presence of glomerular macrophages correlates with endocapillary hypercellularity and sclerosis,33 and loss of renal function.34 Whether or not the identification of these cells using immunohistochemistry might assist pathologists in categorizing lesions, and whether quantifying the number of infiltrating cells is of value as an independent prognostic marker, is currently unknown.
Extracapillary hypercellularity
Extracapillary proliferative lesions, or cellular crescents, are defined by the proliferation of cells in Bowman's space with more than two cell layers (Figure 1). These lesions are present in around one-third of IgA nephropathy biopsies. However, the proportion of glomeruli containing crescents is typically low; crescentic glomerulonephritis, defined as >50% of glomeruli with crescents, is rare in IgA nephropathy, accounting for 1% of biopsies in the Oxford classification cohort17 and 5% in a series from patients with antineutrophil cytoplasmic antibody-positive IgA nephropathy.35 Necrosis, defined as fibrin exudation secondary to capillary rupture with or without karyorrhexis, is a frequent underlying mechanism for extracapillary proliferation and these lesions are, therefore, often seen in combination. Fibrin exudation is poorly visualized with a PAS stain, and requires haematoxylin and eosin staining or a fibrin stain (such as martius scarlet blue) for its reliable identification.17 Masson's trichrome stain might also be useful to identify fibrinoid necrosis, and Jones silver methenamine stain might demonstrate disruptions in the glomerular basement membrane. Necrotizing lesions are uncommon in primary IgA nephropathy, but are more frequent in biopsies in Henoch–Schönlein purpura nephritis.36 A source of diagnostic difficulty and interobserver variation is the distinction between true crescents and 'pseudocrescents' that develop in association with severe podocyte injury. Pseudocrescents are characterised by capillary tuft collapse, epithelial cell hypertrophy and hyperplasia, and protein resorption droplets in the epithelial cytoplasm. Nephrotic syndrome is associated with pseudocresents that might be visible in the same biopsy as necrotizing lesions with true cellular crescents, although they have a different pathogenesis. Both types of lesion are associated with the development of segmental sclerosis.
Segmental glomerulosclerosis
Segmental glomerulosclerosis, defined histologically as occlusion of the lumina of capillaries by extracellular matrix in part of a glomerulus, is a frequent finding in IgA nephropathy (Figure 1). This pathology was present in 76% of biopsies in the Oxford classification cohort, in which patients were selected for proteinuria >0.5 g/l per 24 h.16 Focal segmental sclerosis can be the dominant abnormality and was present in 35% of biopsy samples in one series selected for 'mild IgA nephropathy' with only minimal proliferation.37 In these patients, the morphology might closely resemble that in primary nephrotic FSGS and the distinction between IgA nephropathy and primary FSGS is based on the demonstration of mesangial IgA deposits. Segmental sclerosis might develop as a result of healed necrotizing or segmental proliferative lesions, or reflect podocyte injury as in primary FSGS. Subclassification of segmental sclerosing lesions according to the presence of features associated with podocytopathy and heavy proteinuria (podocyte hypertrophy, protein resorption droplets within podocytes, endocapillary foam cells and glomerular tip lesions) was carried out on the Oxford classification biopsy samples.38 The presence of glomerular tip lesions and podocyte hypertrophy correlated with increased proteinuria at presentation but was not of independent prognostic value.38
Renal tubules and the interstitium
The tubulointerstitium might be near normal early in disease. High levels of proteinuria are associated with protein resorption droplets in tubular epithelial cells and tubular injury. In progressive IgA nephropathy, tubular injury results in a fibroproliferative peritubular response, mononuclear inflammatory cell infiltration and eventually established interstitial fibrosis and tubular atrophy. Tubular atrophy is the most reliable histological marker of an adverse outcome, as discussed in the next section. A low glomerular filtration rate (GFR) at the time a biopsy is carried out usually reflects either established chronic kidney damage (glomerulosclerosis and tubular atrophy) or severe active glomerular lesions (necrosis and/or cellular crescents). Exceptions are patients in which a high level of glomerular haematuria results in widespread red blood cell casts within tubules producing tubular obstruction and acute renal failure even if histological glomerular changes are mild. Glomerular haematuria and red blood cell cast nephropathy might be exacerbated by anticoagulation therapy, resulting in permanent loss of GFR. Thus, treatment with warfarin and antiplatelet agents might be a confounding factor in the outcome of clinical trials in IgA nephropathy.39
Clinicopathological correlations
Many studies performed prior to the publication of the Oxford classification tried to define the clinical relevance of histological lesions in IgA nephropathy.40,41,42,43,44,45,46,47,48,49,50,51,52,53,54 Most of these studies report that, as in other glomerular diseases, the extent of chronic kidney damage (glomerulosclerosis, tubular atrophy and interstitial fibrosis) is the most reliable indicator of renal outcome.41,43,44,45,46,47,48,49,50,51,52,54 By contrast, relatively few report that active proliferative lesions are significantly associated with the development of renal failure. Cellular crescents were of prognostic value in only three studies,40,47,48 endocapillary hypercellularity in one41 and mesangial hypercellularity in two.42,53 An additional consideration when interpreting these data is that histological lesions, and their clinical correlations, might differ between IgA nephropathy diagnosed in biopsies performed in children and those in adults.55 The severity of chronic tubulointerstitial damage correlates not only with renal function or renal survival at the end of follow-up, but also with clinical parameters (such as proteinuria) at the time a biopsy is taken. This correlation reflects a pathogenetic link between urinary leak of proteins, including cytokines, from injured glomeruli, resulting in tubular epithelial injury and activation, which in turn trigger interstitial inflammation and the fibrotic response.
In most instances, analysis of tubulointerstitial damage is carried out semiquantitatively on tissue sections, with estimates of the percentage of renal cortex with tubular atrophy or fibrosis. The most common method is that used for transplant biopsies in which the extent of tubular atrophy and interstitial fibrosis is categorized into three groups (<25%, 25–50% and >50% of the cortical area). Interobserver agreement between pathologists might be low when this semiquantitative or 'eye-balling' approach is used to interpret tubulointerstitial changes in renal transplant biopsies.27 By contrast, the interobserver reproducibility of quantifying percentage tubular atrophy in the Oxford classification study of IgA nephropathy was very high (ICC 0.79),17 despite the lack of a prescribed scoring system among members of the working group. Different pathologists had their own methods and frequently used different stains to assess chronic tubulointerstitial damage. This discrepancy in interobserver reproducibility might reflect differences in pathogenesis and, therefore, the pattern of the tubulointerstitial injury between IgA nephropathy and transplant pathology. In IgA nephropathy, as in other chronic glomerular diseases, tubular atrophy is typically multifocal, with sharply demarcated areas of established atrophy that are usually easy to quantify by eye balling the tissue sections. By contrast, primary tubular pathologies such as tubulointerstitial nephritis and T cell-mediated rejection in transplants result in diffuse tubular atrophy and interstitial fibrosis that are difficult to reliably quantify. Methodological issues such as these should be considered when interpreting the relevance of many retrospective clinicopathological studies to management of individual patients.
An alternative approach to traditional histological measures of chronic kidney damage is to quantify cellular infiltrates. Several studies have demonstrated that interstitial fibrosis in IgA nephropathy is associated with increased numbers of mast cells,56 macrophages57 and T cells.58 Quantification of these infiltrates might be a superior histological marker of progressive renal injury than measurement of fibrosis.
In most studies serum creatinine, creatinine clearance or renal failure at the end of follow up is used as the end point to correlate with pathological findings. Renal function at a single time point reflects the product of both reversible and irreversible damage—that is, disease activity (grade) and chronicity (stage) at the time of biopsy. The later in the course of the disease that a biopsy is carried out, and the later diagnosis of IgA nephropathy is made, the shorter the time to ESRD and renal replacement therapy. Thus, most studies unsurprisingly indicate that the presence of chronic sclerosing lesions is the strongest predictor of renal survival. More important for the management of patients is to identify those individuals with active, progressive disease and, in particular, those who will benefit from immunosuppressive therapy. If histological changes are plotted against the slope of 1/serum creatinine (μm/l), the severity of proliferative lesions predicts the rate of loss of renal function, as discussed in the next section.
Histological classification
A number of attempts have been made to provide a histological classification of IgA nephropathy over the past 40 years. The first published was Meadow's classification of Henoch–Schönlein purpura nephritis.59 This classification was later adapted to IgA nephropathy by Lee et al.60 (Table 1). Another schema was developed by Haas,29 which uses elements of the WHO and International Society of Nephrology/Renal Pathology Society classification of lupus nephritis (Box 1). All of these schema use the format of discrete categories or 'classes' defined by a combination of morphological changes seen in biopsies. The strength of these schemas lies in their simplicity to use in routine practice. The schemas developed by Lee et al.60 and Haas29 are of clinical relevance in a number of series, but there is little evidence that either schema is superior to only scoring chronic tubulointerstitial damage or even a combination of clinical parameters, such as blood pressure and proteinuria.31
The schemas described have several limitations. First, a combination of disease activity (for example, cellularity) and chronicity markers (for example, sclerosis) in the same categories is not optimal for differentiating patients in whom the disease might respond to immunosuppressive therapy and patients with irreversible renal injury. The classification by Lee et al.60 uses descriptive terms, such as 'mostly', 'minor', 'occasional', 'localised' and 'frequent', which limits the interobserver reproducibility of this schema. The classification by Haas29 does not differentiate between mesangial and endocapillary proliferative lesions, which might be of prognostic value. The presence and extent of extracapillary proliferation (crescents), which have been demonstrated in several series to be associated with clinically aggressive disease, is not taken into account. Even in the study by Haas,29 patients with IgA nephropathy and class III histopathological changes and cellular crescents had lower rates of renal survival than patients with class IV changes without crescents.
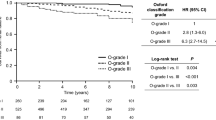
A Japanese research group developed a clinicopathological scoring system for patients with IgA nephropathy with the aim of defining their risk of developing ESRD.61 A prognostic scoring system based on eight clinical and pathological variables accurately predicted renal outcome in a prospective cohort of 207 patients with IgA nephropathy.61 In another study, a lumped classification (placing multiple lesions in the same category) was introduced, based on multivariate logistic regression analysis of a retrospective biopsy sample series from 287 patients with isolated haematuria or mild proteinuria.62 Four histological 'grades' were established, defined as <25%, 25–49%, 50–74% and ≥75% glomeruli with cellular, fibrocellular or fibrous crescents, segmental sclerosis or global sclerosis. This system predicts the risk of progression to ESRD, which developed in 68% of patients with changes of histological grade 4 (OR 27.6) compared with 7% of patients with changes of histological grade 1 (OR 1.0). However, as with other similar schemas, owing to the combination of histological markers of disease activity and chronicity, this system is unlikely to aid in guiding therapy; glomerulosclerosis and cellular crescents were added together to determine histological 'grade', despite their very different therapeutic implications.
The Oxford classification
In 2005, an international working group of over 40 nephrologists and nephropathologists was formed with the aim of developing an evidence-based and reproducible pathological classification of IgA nephropathy. This work led to the publication of the Oxford classification in 2009.16,17 The schema was based on a retrospective clinicopathological study of 265 patients. The cohort included adults and children of various ethnicities, selected on the basis of a detailed clinical dataset, proteinuria >0.5 g/l per 24 h, GFR >30 ml/min/1.73 m2 at the time of diagnosis, and follow-up duration of >12 months. Patients who progressed to ESRD within 1 year of presentation, and those diagnosed with Henoch–Schönlein purpura nephritis, were excluded. The two principle end points were the rate of loss of renal function (that is, the slope of the estimated glomerular filtration rate [eGFR]) and renal survival (ESRD or a 50% decrease in the GFR) at the end of follow up.
All biopsies were scored independently by at least three pathologists, producing a detailed dataset including 25 histological variables.17 Subsequently, the variables were simplified on the basis of interobserver reproducibility, independence from other histological lesions and prognostic value for one or both clinical end points. What resulted was a scoring system, not a classification. Histological variables that met the above criteria were of independent prognostic significance; analysis of the data did not support the generation of discrete categories or classes, as in the schema described in the previous section.16
Six histological variables were found to be both reproducible in terms of interobserver scoring and independent of other lesions: mesangial hypercellularity, endocapillary hypercellularity, cellular or fibrocellular crescents, segmental sclerosis, tubular atrophy/interstitial fibrosis and arteriosclerosis. Three of these variables were independent predictors of either one or both end points in a multivariate analysis that included clinical variables at the time of diagnosis (GFR, proteinuria and blood pressure) and follow up (proteinuria and blood pressure). Endocapillary hypercellularity showed an interaction with treatment; patients whose biopsies showed endocapillary hypercellularity had improved outcomes if they had received immunosuppressive therapy. Thus, four histological lesions were included in the final schema. Receiver operating curve analysis was used to determine the threshold for the percentage of glomeruli or renal cortex showing these changes, to generate the MEST criteria (mesangial hypercellularity [M], endocapillary hypercellularity [E], segmental glomerulosclerosis [S] and tubular atrophy/interstitial fibrosis [T]; Table 2).16
One controversial aspect of the Oxford classification is the omission of crescents, which were not of significant independent prognostic value. This finding might be an accurate conclusion; only a minority of clinicopathological studies indicate that cellular crescents predict the outcome in IgA nephropathy. However, there are sources of bias in the Oxford classification study that warrant consideration. First, patients with low eGFR at presentation, and patients who progressed to ESRD within 12 months of diagnosis, were excluded. Potentially, patients with the most aggressive crescentic disease were removed from the study, reflected in the observation that even though 45% of biopsies had cellular crescents, the proportion of glomeruli containing crescents was low (median of 9%).16 Second, 29% of patients were treated with steroids or cytotoxic agents and those whose biopsies had crescents were more likely to have received immunosuppression. Treatment bias is a confounding factor in many other retrospective clinicopathological studies published before and after the Oxford classification. Very few offer insight on the impact of histological findings on the natural history of IgA nephropathy.
Validating the Oxford classification
The prognostic value of the MEST criteria requires validation in other patient groups, particularly in patients who were excluded from the Oxford classification cohort. In the 4 years since the Oxford classification was published, 17 validation studies have been reported28,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77 using similar methodology, including retrospective clinicopathological studies uncontrolled for therapy. The studies include both children and adults, and patients of various ethnicities, including Asian, European and American.
In general, the validation studies support some but not all of the findings in the Oxford classification study. A majority confirm the prognostic value of the T-score in multivariate analysis,28,63,64,65,67,68,70,71,72,73,74,76,77 and several also confirm that the M-score and/or S-score are independent predictors of outcome.28,62,63,64,65,67,69,72,73 Most studies use renal survival as the clinical end point and it is, therefore, not surprising that tubular atrophy/interstitial fibrosis (T-score) is the strongest histological predictor of outcome, as a high T-score reflects advanced disease at the time of diagnosis. All but three of the validation studies found the E-score was not associated with clinical outcomes. The lack of prognostic value of endocapillary hypercellularity in most studies might reflect treatment bias; immunosuppressive therapy was frequent in all but two studies.63,78 A median 35% of patients in these studies received steroids and in all of those with the relevant analysis there was evidence of treatment bias; patients whose biopsies had endocapillary proliferation and crescents were most likely to have been treated with steroids. The findings of the VALIGA study28 demonstrated the effect of therapy on the prognostic value of histological scoring; adding the M-score, S-score and T-score to clinical variables significantly improved the ability to predict progression only in those patients who did not receive immunosuppression. In two studies, in Paris63 and Oxford,78 there was little or no immunosuppression with 0% and 5% of patients treated with steroids. The rate of loss of renal function was an end point in the multivariate analysis, and in both studies the E-score was significantly associated with rate of disease progression.
Three validation studies, respectively, in Japan,64 China4 and Europe28 included 702, 1,026 and 1,147 patients. A subgroup analysis was possible due to the large sample sizes, with the aim of determining the effect of histological findings on outcomes in patients with various clinical presentations. The study by Katafuchi et al.64 indicated that glomerular crescents were not of prognostic value in the 416 patients that met the same inclusion criteria as in the Oxford classification cohort, but were in 218 patients who did not meet these criteria. The latter group included patients with rapidly progressive disease. The optimal cut-off for dichotomization of the percentage of glomeruli containing crescents was 6.8% (rather than crescents being present or absent) for the purpose of identifying patients at increased risk of disease progression. The prognostic value of the M-score, S-score and T-score was verified in the VALIGA study. These scores were independent predictors of one or both clinical outcomes (rate of loss of renal function and ESRD or 50% loss of renal function) in a multivariate analysis that included clinical variables. The E-score was not of prognostic value in the whole cohort, but in patients with proteinuria <0.5 g/l per 24 h at presentation, endocapillary hypercellularity was the only histological lesion that predicted renal outcome in a univariate analysis.28 M- and E-scores in this group of patients with 'good prognosis' clinical features were significantly associated with progression to higher levels of proteinuria, with hazard ratios of 4.1 and 4.2, respectively, for developing proteinuria >2 g/l per 24 h. A single-centre study75 of 141 patients with good prognosis IgA nephropathy (eGFR >60 ml/min/1.73 m2 and minimal proteinuria), reported a low frequency of both proliferative glomerular lesions and sclerosis. Segmental glomerulosclerosis was the only lesion predictive of a >50% increase in serum creatinine.
Predicting response to therapy
In addition to providing prognostic information, renal biopsies might potentially be used to guide therapy. Two lines of evidence suggest that certain histological lesions in IgA nephropathy are responsive to treatment with steroids or cytotoxic agents and that this impacts on clinical outcome.
A small number of studies have carried out two biopsies, one before and one after treatment, demonstrating a reduction in active proliferative lesions. In one study,79 16 patients with a vasculitic or crescentic pattern of IgA nephropathy were treated with prednisolone and cyclophosphamide or azathioprine. A repeat biopsy at the end of treatment showed a reduction in the extent of active vasculitic lesions (segmental necrosis and extracapillary proliferation) that were present in a median 17.5% and 0% of glomeruli in the first and second biopsies, respectively. In another study,80 12 patients whose initial biopsies showed crescentic IgA nephropathy were treated with methylprednisolone and cyclophosphamide followed by a tapered course of prednisolone. At the end of therapy, biopsies showed a marked reduction in activity index using a revised NIH schema. In a Japanese series of 35 patients treated with tonsillectomy and pulse methylprednisolone followed by a tapered dose of prednisolone for 12 months, crescents were present in 91% of the initial biopsies and 0% of the second biopsies carried out at the end of treatment.11 This study indicated that it is not only vasculitic (necrotizing) and crescentic lesions that are responsive to therapy including steroids; there was also a highly significant reduction in mesangial proliferation score between the first and second biopsies.
Most retrospective clinicopathological studies with appropriate statistical analyses demonstrate an interaction between therapy and endocapillary hypercellularity and/or cellular crescents. In the Oxford classification cohort, patients whose biopsies showed endocapillary hypercellularity had an improved outcome if they were treated with steroids.16 Similar findings were reported from validation studies in America67 and China,72 and two other studies demonstrated a similar interaction between therapy and crescents.64,67 Tomiyoshi et al.,81 in a small study of 28 patients, demonstrated that necrosis within glomeruli and cellular crescents predict the response to steroid therapy. Studies such as these indicate a role for immunosuppressive therapy in patients with endocapillary proliferative or crescentic pattern IgA nephropathy. What is required now is supporting evidence from prospective randomized controlled trials (RCTs).
Limited conclusions can be drawn from published RCT data regarding which histological lesions predict a response to therapy. Most studies have either not had sufficient numbers to address this question,53,82 or the assessment of histological data has been suboptimal. In several RCTs, active proliferative and chronic sclerosing lesions have been combined in a lumped classification that has prognostic value but does not provide information on which individual lesions can be used to guide therapy.83,84 A small RCT of sirolimus in patients with IgA nephropathy, which included a second biopsy carried out at 1 year, indicated there was a significant reduction in mesangial and endocapillary proliferation in patients in the treatment arm compared with those in the control arm.85
Recurrent IgA nephropathy
Recurrence of IgA nephropathy after transplantation is common, occurring in approximately one-third of patients. Glomerular IgA deposits might be an incidental finding and not the primary cause of graft dysfunction that triggered a biopsy. Frequently, however, there is evidence from clinical features (haematuria/proteinuria) and histological changes (proliferative glomerular lesions) that IgA deposits result in kidney injury. The median time to recurrence is 3–4 years and IgA nephropathy in the transplanted kidney is an important cause of late graft failure.86,87 In general, there is a lower frequency of active proliferative glomerular lesions but more severe chronic damage in posttransplant IgA nephropathy when compared with native disease.88 The low frequency of proliferative glomerular lesions in transplanted kidneys might reflect the effect of immunosuppressive therapy post-transplantation and the chronic damage might result from other mechanisms, such as ischaemic injury and graft rejection.
Studies of protocol biopsies demonstrate that subclinical glomerular IgA deposition is common; in a series of 65 protocol biopsy samples, one group89 reported a recurrence rate of 32% and that glomerular IgA deposits were only associated with urinary abnormalities in half of these patients. The interval between immunological and clinical recurrence, however, might be short. In a series of 35 patients with IgA nephropathy who received transplants, the presence of subclinical IgA deposits in 1-month protocol biopsy samples was associated with early clinical recurrence.8
Limited data is available on the histological predictors of clinical outcome in posttransplant IgA nephropathy. One study has investigated the prognostic value of the Oxford classification in post-transplant IgA nephropathy.90 Endocapillary hypercellularity, segmental sclerosis and tubular atrophy/interstitial fibrosis, but not mesangial hypercellularity, were predictive of graft failure in 114 patients with IgA nephropathy diagnosed in transplant biopsies (irrespective of the primary cause of disease).
Conclusions and future challenges
The principle underlying the Oxford classification from the outset was that it should have a strong evidence base, rather than introduce arbitrary divisions and diagnostic criteria. The classification was expected to evolve as data from retrospective studies and clinical trials accumulate. Future revisions and modifications are intended in the light of new developments. The inaugural Oxford conference on IgA nephropathy is to be held with the specific aims of reviewing the Oxford classification and making modifications according to the evidence.
A major challenge that remains, however, is to improve interobserver reproducibility in the scoring of histological lesions in biopsy samples. Findings from the VALIGA study highlight differences in the ways pathologists score active proliferative lesions, and that these discrepancies can have a major effect on the prognostic value of the pathology report. A need exists for robust guidelines in the assessment of glomerular cellularity, including practical advice, which could be provided by an internet-based training programme. A prognostic modelling system incorporating histological, clinical and biomarker data might also aid in the management of patients. Evidence is accumulating that the measurement of serum and urinary biomarkers is of value in the assessment of patients. A prognostic modelling system that combines multiple types of data has the potential to improve prognostication and also to accurately identify patients who will benefit most from therapeutic interventions.
Review criteria
Referenced articles were identified by PubMed searches for original full-text articles on IgA nephropathy. The search terms used were variously “IgA nephropathy”, “histology”, “classification” and “prognosis”, alone and in combination. All articles identified were in English. I also searched the reference lists of identified articles for further relevant papers.
References
Seedat, Y. K., Nathoo, B. C., Parag, K. B., Naiker, I. P. & Ramsaroop, R. IgA nephropathy in blacks and Indians of Natal. Nephron 50, 137–141 (1988).
Schena, F. P. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am. J. Med. 89, 209–215 (1990).
D'Amico, G. et al. Idiopathic IgA mesangial nephropathy. Clinical and histological study of 374 patients. Medicine (Baltimore) 64, 49–60 (1985).
Zeng, C. H. et al. A multicenter application and evaluation of the Oxford Classification of IgA nephropathy in adult Chinese patients. Am. J. Kidney Dis. 60, 812–820 (2012).
Lai, K. N. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 8, 275–283 (2012).
Suzuki, H. et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Invest. 119, 1668–1677 (2009).
Novak, J. et al. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 67, 504–513 (2005).
Roberts, I. S. D., Price, N., Sarkar, E. & Winearls, C. Subclinical recurrence of IgA nephropathy following renal transplantation: evidence from early protocol biopsies. J. Pathol. 210 (Suppl. S1), 74A (2006).
Waldherr, R., Rambausek, M., Duncker, W. D. & Ritz, E. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol. Dial. Transplant. 4, 943–946 (1989).
Valentijn, R. M. et al. Circulating and mesangial secretory component-binding IgA-1 in primary IgA nephropathy. Kidney Int. 26, 760–766 (1984).
Hotta, O., Furuta, T., Chiba, S., Tomioka, S. & Taguma, Y. Regression of IgA nephropathy: a repeat biopsy study. Am. J. Kidney Dis. 39, 493–502 (2002).
Bellur, S. S., Troyanov, S., Cook, H. T. & Roberts, I. S. Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol. Dial. Transplant. 26, 2533–2536 (2011).
Andreoli, S. P., Yum, M. N. & Bergstein, J. M. Significance of glomerular basement membrane deposition of IgA. Am. J. Nephrol. 6, 28–33 (1986).
Yoshimura, M. et al. Significance of IgA deposits on the glomerular capillary walls in IgA nephropathy. Am. J. Kidney Dis. 9, 404–409 (1987).
D'Amico, G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin. Nephrol. 24, 179–196 (2004).
Cattran, D. C. et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 76, 534–545 (2009).
Roberts, I. S. et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 76, 546–556 (2009).
Vangelista, A., Frasca, G. M., Mondini, S. & Bonomini, V. Idiopathic mesangial IgA nephropathy: immunohistological features. Contrib. Nephrol. 40, 167–173 (1984).
Jennette, J. C. The immunohistology of IgA nephropathy. Am. J. Kidney Dis. 12, 348–352 (1988).
Jennette, J. C., Olson, J. L., Schwartz, M. M. & Silva, F. G. Heptinstall's Pathology of the Kidney 6th edn Vol. 1, 424–487 (Lippincott Williams & Wilkins, 2007).
Eison, T. M. et al. Association of IgG co-deposition with serum levels of galactose-deficient IgA1 in pediatric IgA nephropathy. Clin. Nephrol. 78, 465–469 (2012).
Nieuwhof, C., Kruytzer, M., Frederiks, P. & van Breda Vriesman, P. J. Chronicity index and mesangial IgG deposition are risk factors for hypertension and renal failure in early IgA nephropathy. Am. J. Kidney Dis. 31, 962–970 (1998).
Wada, Y. et al. Clinical significance of IgG deposition in the glomerular mesangial area in patients with IgA nephropathy. Clin. Exp. Nephrol. 17, 73–82 (2013).
Kim, S. J. et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS ONE 7, e40495 (2012).
Maeng, Y. I. et al. Glomerular and tubular C4d depositions in IgA nephropathy: relations with histopathology and with albuminuria. Int. J. Clin. Exp. Pathol. 6, 904–910 (2013).
Espinosa, M. et al. Mesangial C4d deposition: a new prognostic factor in IgA nephropathy. Nephrol. Dial. Transplant. 24, 886–891 (2009).
Furness, P. N., Taub N. & Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 60, 1998–2012 (2001).
Coppo, R. et al. The predictive value of the Oxford classification of IgA nephropathy in cohorts with different presentations and receiving different treatments: the VALIGA study. Kidney Int. http://dx.doi.org/10.1038/ki.2014.63.
Haas, M. Histological subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am. J. Kidney Dis. 29, 829–842 (1997).
Hall, Y. N., Fuentes, E. F., Chertow, G. M. & Olson, J. L. Race/ethnicity and disease severity in IgA nephropathy. BMC Nephrol. 5, 10 (2004).
Bartosik, L. P., Lajoie, G., Sugar, L. & Cattran, D. C. Predicting progression in IgA nephropathy. Am. J. Kidney Dis. 38, 728–735 (2001).
Wu, Q., Tanaka, H., Hirukawa, T., Endoh, M. & Fukagawa, M. Characterization and quantification of proliferating cell patterns in endocapillary proliferation. Nephrol. Dial. Transplant. 27, 3234–3241 (2012).
Ikezumi, Y. et al. Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology 58, 198–210 (2011).
Yang, N. et al. Local macrophage proliferation in human glomerulonephritis. Kidney Int. 54, 143–151 (1998).
Haas, M. et al. ANCA-associated crescentic glomerulonephritis with mesangial IgA deposits. Am. J. Kidney Dis. 36, 709–718 (2000).
Szeto, C. C. et al. Grading of acute and chronic renal lesions in Henoch-Schönlein purpura. Mod. Pathol. 14, 635–640 (2001).
Weber, C. L., Rose, C. L. & Magil, A. B. Focal segmental glomerulosclerosis in mild IgA nephropathy: a clinical-pathologic study. Nephrol. Dial. Transplant. 24, 483–488 (2009).
Roberts, I. S., Bellur, S. S. & Cook, H. T. Subclassification of focal segmental glomerulosclerosis in IgA nephropathy: is it of clinical value? Evidence from the Oxford classification cohort. J. Am. Soc. Nephrol. 21, 420A (2010).
Brodsky, S. V., Rovin, B. H. & Hebert, L. A. Benefit of cyclophosphamide therapy in IgA nephritis may have been obscured by warfarin-related nephropathy in the randomized trials in which warfarin and dipyridamole were used in combination with cyclophosphamide. Nephrol. Dial. Transplant. 27, 475–477 (2012).
Boyce, N. W., Holdsworth, S. R., Thomson, N. M. & Atkins R. C. Clinicopathological associations in mesangial IgA nephropathy. Am. J. Nephrol. 6, 246–252 (1986).
D'Amico, G. et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q. J. Med. 59, 363–378 (1986).
Rekola, S., Bergstrand, A. & Bucht, H. IgA nephropathy: a retrospective evaluation of prognostic indices in 176 patients. Scand. J. Urol. Nephrol. 23, 37–50 (1989).
Bogenschutz, O. et al. IgA nephritis: on the importance of morphological and clinical parameters in the long-term prognosis of 239 patients. Am. J. Nephrol. 10, 137–147 (1990).
Okada, H., Suzuki, H., Konishi, K., Sakaguchi, H. & Saruta, T. Histological alterations in renal specimens as indicators of prognosis of IgA nephropathy. Clin. Nephrol. 37, 235–238 (1992).
Ibels, L. S. & Gyory, A. Z. IgA nephropathy: analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine (Baltimore) 73, 79–102 (1994).
Katafuchi, R. et al. An important role of glomerular segmental lesions on progression of IgA nephropathy: a multivariate analysis. Clin. Nephrol. 41, 191–198 (1994).
Hogg, R. J. et al. Prognostic indicators in children with IgA nephropathy--report of the Southwest Pediatric Nephrology Study Group. Pediatr. Nephrol. 8, 15–20 (1994).
Freese, P., Norden, G. & Nyberg, G. Morphologic high-risk factors in IgA nephropathy. Nephron 79, 420–425 (1998).
Vleming, L. J. et al. Histomorphometric correlates of renal failure in IgA nephropathy. Clin. Nephrol. 49, 337–344 (1998).
Daniel, L. et al. Tubular lesions determine prognosis of IgA nephropathy. Am. J. Kidney Dis. 35, 13–20 (2000).
Mera, J., Uchida, S. & Nagase, M. Clinicopathologic study on prognostic markers in IgA nephropathy. Nephron 84, 148–157 (2000).
To, K. F., Choi, P. C. & Szeto, C. C. Outcome of IgA nephropathy in adults graded by chronic histological lesions. Am. J. Kidney Dis. 35, 392–400 (2000).
Ballardie, F. W. & Roberts, I. S. D. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J. Am. Soc. Nephrol. 13, 142–148 (2002).
Nozawa, R. et al. Clinicopathological features and the prognosis of IgA nephropathy in Japanese children on long-term observation. Clin. Nephrol. 64, 171–179 (2005).
Ikezumi, Y. et al. Histological differences in new-onset IgA nephropathy between children and adults. Nephrol. Dial. Transplant. 21, 3466–3474 (2006).
Roberts, I. S. D. & Brenchley, P. E. C. Mast cells: the forgotten cells of renal fibrosis. J. Clin. Pathol. 53, 858–862 (2000).
Zhu, G., Wang, Y., Wang, J. & Tay, Y.-C. Significance of CD25 positive cells and macrophages in non-crescentic IgA nephropathy. Renal Failure 28, 229–235 (2006).
Myllymaki, J. M. et al. Severity of tubulointerstitial inflammation and prognosis in immunoglobulin A nephropathy. Kidney Int. 71, 343–348 (2007).
Meadow, S. R. et al. Schonlein-Henoch nephritis. Q. J. Med. 163, 241–258 (1972).
Lee, S.-M. K. et al. IgA nephropathy: morphologic predictors of progressive renal disease. Hum. Pathol. 13, 314–322 (1982).
Wakai, K. et al. A scoring system to predict renal outcome in IgA nephropathy: from a nationwide prospective study. Nephrol. Dial. Transplant. 21, 2800–2808 (2006).
Kawamura, T. et al. A histologic classification of IgA nephropathy for predicting long-term prognosis: emphasis on end-stage renal disease. J. Nephrol. 26, 350–357 (2013).
El Karoui, K. et al. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. II. Light microscopic and clinical studies. Kidney Int. 79, 643–654 (2011).
Katafuchi, R. et al. Validation study of Oxford Classification of IgA nephropathy: the significance of extracapillary proliferation. Clin. J. Am. Soc. Nephrol. 6, 2806–2813 (2011).
Alamartine, E. et al. The use of the Oxford classification of IgA nephropathy to predict renal survival. Clin. J. Am. Soc. Nephrol. 6, 2384–2388 (2011).
Edström Halling, S., Söderberg, M. P. & Berg, U. B. Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification). Nephrol. Dial. Transplant. 27, 715–722 (2012).
Herzenberg, A. M. et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 80, 310–317 (2011).
Kang, S. H. et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol. Dial. Transplant. 27, 252–258 (2012).
Kataoka, H. et al. Overweight and obesity accelerate the progression of IgA nephropathy: prognostic utility of a combination of BMI and histopathological parameters. Clin. Exp. Nephrol. 16, 706–712 (2012).
Lee, H. et al. Validation of the Oxford Classification of IgA nephropathy: a single-center study in Korean adults. Korean J. Intern. Med. 27, 293–300 (2012).
Moriyama, T. et al. Severity of nephrotic IgA nephropathy according to the Oxford classification. Int. Urol. Nephrol. 44, 1177–1184 (2012).
Shi, S. F. et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the Oxford Classification. Clin. J. Am. Soc. Nephrol. 6, 2175–2184 (2011).
Shima, Y. et al. Validity of the Oxford classification of IgA nephropathy in children. Pediatr. Nephrol. 27, 783–792 (2012).
Yau, T., Korbet, S. M., Schwartz, M. M. & Cimbaluk, D. J. The Oxford classification of IgA nephropathy: a retrospective analysis. Am. J. Nephrol. 34, 435–444 (2011).
Gutierrez, E. et al. Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J. Am. Soc. Nephrol. 23, 1753–1760 (2012).
Le, W. et al. Validation of the Oxford Classification of IgA Nephropathy for pediatric patients from China. BMC Nephrol. 13, 158 (2012).
Park, K. S. et al. Comparison of the Haas and the Oxford classifications for prediction of renal outcome in patients with IgA nephropathy. Hum. Pathol. 45, 236–243 (2014).
Chakera, A. et al. Prognostic value of endocapillary proliferation in IgA nephropathy patients with minimal immunosuppression [abstract]. Mod. Pathol. 26 (Suppl. 2), 385A (2013).
Harper, L. et al. Treatment of vasculitic IgA nephropathy. J. Nephrol. 13, 360–366 (2000).
Tumlin, J. A., Lohavichan, V. & Hennigar, R. Crescentic, proliferative IgA nephropathy: clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol. Dial. Transplant. 18, 1321–1329 (2003).
Tomiyoshi, Y. et al. Cellular crescents and segmental glomerular necrosis in IgA nephropathy are indicative of the beneficial effects of corticosteroid therapy. Intern. Med. 40, 862–866 (2001).
Kim, Y. C., Chin, H. J., Koo, H. S. & Kim, S. Tacrolimus decreases albuminuria in patients with IgA nephropathy and normal blood pressure: a double-blind randomized controlled trial of efficacy of tacrolimus on IgA nephropathy. PLoS ONE 8, e71545 (2013).
Manno, C., Torres, D. D., Rossini, M., Pesce, F. & Schena, F. P. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol. Dial. Transplant. 24, 3694–3701 (2009).
Pozzi, C. et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J. Am. Soc. Nephrol. 15, 157–163 (2004).
Cruzado, J. M. et al. Low-dose sirolimus combined with angiotensin-converting enzyme inhibitor and statin stabilizes renal function and reduces glomerular proliferation in poor prognosis IgA nephropathy. Nephrol. Dial. Transplant. 26, 3596–3602 (2011).
Ohmacht, C. et al. Recurrent immunoglobulin A nephritis after renal transplantation; a significant contributor to graft loss. Transplantation 64, 1493 (1997).
Moroni, G. et al. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol. Dial. Transplant. 28, 1305–1314 (2013).
Oka, K. et al. A clinicopathological study of IgA nephropathy in renal transplant recipients: beneficial effect of angiotensin-converting enzyme inhibitor. Nephrol. Dial. Transplant. 15, 689–695 (2000).
Ortiz, F. et al. IgA nephropathy recurs early in the graft when assessed by protocol biopsy. Nephrol. Dial. Transplant. 27, 2553–2558 (2012).
Lim, B. J. et al. Usefulness of Oxford classification in assessing immunoglobulin A nephropathy after transplantation. Transplantation 95, 1491–1497 (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Roberts, I. Pathology of IgA nephropathy. Nat Rev Nephrol 10, 445–454 (2014). https://doi.org/10.1038/nrneph.2014.92
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.92
This article is cited by
-
Treatment and outcome of IgA nephropathy in children from one single center experience
BMC Pediatrics (2023)
-
Established the first clinical prediction model regarding the risk of hyperuricemia in adult IgA nephropathy
International Urology and Nephrology (2023)
-
SARS-CoV-2 infection: a possible trigger for the recurrence of IgA nephropathy after kidney transplantation?
Journal of Nephrology (2023)
-
The association of normal-range serum phosphorus with immunoglobulin A nephropathy progression: a retrospective cohort study
International Urology and Nephrology (2023)
-
Coexistence of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and IgA nephropathy
Immunologic Research (2023)