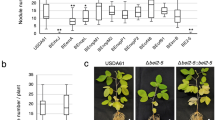
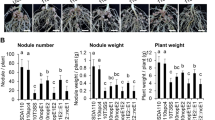
Key Points
-
Symbiotic nitrogen-fixing rhizobial bacteria and leguminous plants have evolved complex signal exchange mechanisms that allow a specific bacterial species to induce its host plant to form invasion structures through which it enters the plant root.
-
Once these invasion structures reach the target cells in the interior of the plant root, the bacteria are endocytosed within a host cell membrane-derived compartment.
-
In the microaerobic environment provided by the host cell, the bacteria differentiate into a specialized form called a bacteroid. The bacteroid form expresses the oxygen-sensitive enzyme nitrogenase that catalyzes the conversion of atmospheric nitrogen to ammonia.
-
The dissection of the bacterial and plant signalling pathways that are involved in each stage of the invasion process has been facilitated by the complete genomic sequencing of Sinorhizobium meliloti and the near complete sequencing of the genome of the model host plant Medicago truncatula.
-
Rhizobial bacteria interact very differently with the plant innate immune system than other groups of bacteria. Rhizobia lack some of the microbial molecular patterns that provoke plant defence responses. Additionally, legume plants differ from other plant families in that they lack the ability to perceive and respond defenceively to other microbial molecular patterns.
-
Symbiotic rhizobial bacteria are similar to pathogenic bacteria such as Brucella spp, in that they both form chronic infections of eukaryotic cells within a host-derived membrane compartment, and require some of the same bacterial factors for survival within the host. These factors include the correct structure of the lipopolysaccharide core and lipid A, presence of cyclic β-glucans, and a common bacterial regulatory circuitry.
Abstract
Nitrogen-fixing rhizobial bacteria and leguminous plants have evolved complex signal exchange mechanisms that allow a specific bacterial species to induce its host plant to form invasion structures through which the bacteria can enter the plant root. Once the bacteria have been endocytosed within a host-membrane-bound compartment by root cells, the bacteria differentiate into a new form that can convert atmospheric nitrogen into ammonia. Bacterial differentiation and nitrogen fixation are dependent on the microaerobic environment and other support factors provided by the plant. In return, the plant receives nitrogen from the bacteria, which allows it to grow in the absence of an external nitrogen source. Here, we review recent discoveries about the mutual recognition process that allows the model rhizobial symbiont Sinorhizobium meliloti to invade and differentiate inside its host plant alfalfa (Medicago sativa) and the model host plant barrel medic (Medicago truncatula).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Perret, X., Staehelin, C. & Broughton, W. J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 64, 180–201 (2000).
Barnett, M. J. & Fisher, R. F. Global gene expression in the rhizobial–legume symbiosis. Symbiosis 42, 1–24 (2006).
Peck, M. C., Fisher, R. F. & Long, S. R. Diverse flavonoids stimulate NodD1 binding to nod gene promoters in Sinorhizobium meliloti. J. Bacteriol. 188, 5417–5427 (2006).
Oldroyd, G. E. & Downie, J. A. Calcium, kinases and nodulation signalling in legumes. Nature Rev. Mol. Cell Biol. 5, 566–576 (2004).
Cook, D. R. Unraveling the mystery of Nod factor signaling by a genomic approach in Medicago truncatula. Proc. Natl Acad. Sci. USA 101, 4339–4340 (2004).
Geurts, R., Fedorova, E. & Bisseling, T. Nod factor signaling genes and their function in the early stages of Rhizobium infection. Curr. Opin. Plant Biol. 8, 346–352 (2005).
Oldroyd, G. E. & Downie, J. A. Nuclear calcium changes at the core of symbiosis signalling. Curr. Opin. Plant Biol. 9, 351–357 (2006).
Timmers, A. C., Auriac, M. C. & Truchet, G. Refined analysis of early symbiotic steps of the Rhizobium–Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 126, 3617–3628 (1999). The definitive microscopy study of plant cytological changes during infection thread formation.
Sieberer, B. J., Timmers, A. C. & Emons, A. M. Nod factors alter the microtubule cytoskeleton in Medicago truncatula root hairs to allow root hair reorientation. Mol. Plant Microbe Interact. 18, 1195–1204 (2005).
Cardenas, L. et al. The role of nod factor substituents in actin cytoskeleton rearrangements in Phaseolus vulgaris. Mol. Plant Microbe Interact. 16, 326–334 (2003).
de Ruijter, N. C. A., Bisseling, T. & Emons, A. M. C. Rhizobium Nod factors induce an increase in subapical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol. Plant Microbe Interact. 12, 829–832 (1999).
Esseling, J. J., Lhuissier, F. G. & Emons, A. M. Nod factor-induced root hair curling: continuous polar growth towards the point of nod factor application. Plant Physiol. 132, 1982–1988 (2003). Demonstrated that Nod factor has a directional effect on root hair polar tip growth.
Gage, D. J. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68, 280–300 (2004).
Foucher, F. & Kondorosi, E. Cell cycle regulation in the course of nodule organogenesis in Medicago. Plant Mol. Biol. 43, 773–786 (2000).
Heidstra, R. et al. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium–legume interaction. Development 124, 1781–1787 (1997).
Wasson, A. P., Pellerone, F. I. & Mathesius, U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18, 1617–1629 (2006).
Amor, B. B. et al. The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 34, 495–506 (2003). Identified an M. truncatula mutant that was completely devoid of responses to Nod factor.
Arrighi, J. F. et al. The Medicago truncatula lysine motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 142, 265–279 (2006). Cloned the NFP -encoded Nod factor receptor and demonstrated that it has a role in infection thread formation.
Limpens, E. et al. LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302, 630–633 (2003). Identified and cloned the MtLYK3 and MtLYK4 Nod factor receptors.
Ane, J. M. et al. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303, 1364–1367 (2004).
Riely, B. K., Lougnon, G., Ane, J. M. & Cook, D. R. The symbiotic ion channel homolog DMI1 is localized in the nuclear membrane of Medicago truncatula roots. Plant J. 49, 208–216 (2007).
Mitra, R. M. et al. A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning. Proc. Natl Acad. Sci. USA 101, 4701–4705 (2004).
Levy, J. et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303, 1361–1364 (2004).
Gleason, C. et al. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 441, 1149–1152 (2006).
Esseling, J. J., Lhuissier, F. G. & Emons, A. M. A nonsymbiotic root hair tip growth phenotype in NORK-mutated legumes: implications for nodulation factor-induced signaling and formation of a multifaceted root hair pocket for bacteria. Plant Cell 16, 933–944 (2004).
Kalo, P. et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308, 1786–1789 (2005).
Smit, P. et al. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science 308, 1789–1791 (2005).
Charron, D., Pingret, J. L., Chabaud, M., Journet, E. P. & Barker, D. G. Pharmacological evidence that multiple phospholipid signaling pathways link Rhizobium nodulation factor perception in Medicago truncatula root hairs to intracellular responses, including Ca2+ spiking and specific ENOD gene expression. Plant Physiol. 136, 3582–3593 (2004).
Dickstein, R., Bisseling, T., Reinhold, V. N. & Ausubel, F. M. Expression of nodule-specific genes in alfalfa root nodules blocked at an early stage of development. Genes Dev. 2, 677–687 (1988). First demonstrated that cyclic- β -glucan mutants and exopolysaccharide mutants of S. meliloti lack infection threads.
Dylan, T., Nagpal, P., Helinski, D. R. & Ditta, G. S. Symbiotic pseudorevertants of Rhizobium meliloti ndv mutants. J. Bacteriol. 172, 1409–1417 (1990).
Yao, S. Y. et al. Sinorhizobium meliloti ExoR and ExoS proteins regulate both succinoglycan and flagellum production. J. Bacteriol. 186, 6042–6049 (2004).
Cheng, H. P. & Walker, G. C. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 180, 20–26 (1998).
Zhang, X. S. & Cheng, H. P. Identification of Sinorhizobium meliloti early symbiotic genes by use of a positive functional screen. Appl. Environ. Microbiol. 72, 2738–2748 (2006).
Klein, S., Hirsch, A. M., Smith, C. A. & Signer, E. R. Interaction of nod and exo Rhizobium meliloti in alfalfa nodulation. Mol. Plant Microbe Interact. 1, 94–100 (1988).
Gage, D. J. Analysis of infection thread development using Gfp- and DsRed-expressing Sinorhizobium meliloti. J. Bacteriol. 184, 7042–7046 (2002). Demonstrated that only bacteria near the tip of an infection thread proliferate and progress into plant tissue.
Pellock, B. J., Cheng, H. P. & Walker, G. C. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol. 182, 4310–4318 (2000).
Glazebrook, J. & Walker, G. C. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell 56, 661–672 (1989).
Reinhold, B. B. et al. Detailed structural characterization of succinoglycan, the major exopolysaccharide of Rhizobium meliloti Rm1021. J. Bacteriol. 176, 1997–2002 (1994).
Cheng, H. P. & Walker, G. C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180, 5183–5191 (1998). Showed that succinoglycan must be succinylated and acetylated to efficiently facilitate infection thread formation.
York, G. M. & Walker, G. C. The succinyl and acetyl modifications of succinoglycan influence susceptibility of succinoglycan to cleavage by the Rhizobium meliloti glycanases ExoK and ExsH. J. Bacteriol. 180, 4184–4191 (1998).
Laus, M. C., van Brussel, A. A. & Kijne, J. W. Exopolysaccharide structure is not a determinant of host-plant specificity in nodulation of Vicia sativa roots. Mol. Plant Microbe Interact. 18, 1123–1129 (2005).
Ardourel, M. et al. Rhizobium meliloti lipooligosaccharide nodulation factors: different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell 6, 1357–1374 (1994).
Kuppusamy, K. T. et al. LIN, a Medicago truncatula gene required for nodule differentiation and persistence of rhizobial infections. Plant Physiol. 136, 3682–3691 (2004).
Middleton, P. H. et al. An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell 19, 1221–1234 (2007).
Sieberer, B. J., Ketelaar, T., Esseling, J. J. & Emons, A. M. Microtubules guide root hair tip growth. New Phytol. 167, 711–719 (2005).
Esseling, J. J. & Emons, A. M. Dissection of Nod factor signalling in legumes: cell biology, mutants and pharmacological approaches. J. Microsc. 214, 104–113 (2004).
Vassileva, V. N., Kouchi, H. & Ridge, R. W. Microtubule dynamics in living root hairs: transient slowing by lipochitin oligosaccharide nodulation signals. Plant Cell 17, 1777–1787 (2005).
Brewin, N. J. Plant cell wall remodelling in the Rhizobium–Legume symbiosis. Crit. Rev. Plant Sci. 23, 293–316 (2004).
Vandenbosch, K. A. et al. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J. 8, 335–341 (1989). The first immunological detection of antigens localizing to the infection thread.
Rathbun, E. A., Naldrett, M. J. & Brewin, N. J. Identification of a family of extensin-like glycoproteins in the lumen of rhizobium-induced infection threads in pea root nodules. Mol. Plant Microbe Interact. 15, 350–359 (2002).
Santos, R., Herouart, D., Sigaud, S., Touati, D. & Puppo, A. Oxidative burst in alfalfa–Sinorhizobium meliloti symbiotic interaction. Mol. Plant–Microbe Interact. 14, 86–89 (2001).
Abramovitch, R. B., Anderson, J. C. & Martin, G. B. Bacterial elicitation and evasion of plant innate immunity. Nature Rev. Mol. Cell Biol. 7, 601–611 (2006).
Sigaud, S., Becquet, V., Frendo, P., Puppo, A. & Herouart, D. Differential regulation of two divergent Sinorhizobium meliloti genes for HPII-like catalases during free-living growth and protective role of both catalases during symbiosis. J. Bacteriol. 181, 2634–2639 (1999).
Jamet, A., Sigaud, S., Van de Sype, G., Puppo, A. & Herouart, D. Expression of the bacterial catalase genes during Sinorhizobium meliloti–Medicago sativa symbiosis and their crucial role during the infection process. Mol. Plant Microbe Interact. 16, 217–225 (2003).
Davies, B. W. & Walker, G. C. Disruption of sitA compromises Sinorhizobium meliloti for manganese uptake required for protection against oxidative stress. J. Bacteriol. 189, 2101–2109 (2007).
Jamet, A., Kiss, E., Batut, J., Puppo, A. & Herouart, D. The katA catalase gene is regulated by OxyR in both free-living and symbiotic Sinorhizobium meliloti. J. Bacteriol. 187, 376–381 (2005).
Barloy-Hubler, F., Cheron, A., Hellegouarch, A. & Galibert, F. Smc01944, a secreted peroxidase induced by oxidative stresses in Sinorhizobium meliloti 1021. Microbiology 150, 657–664 (2004).
Gonzalez-Rizzo, S., Crespi, M. & Frugier, F. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18, 2680–2693 (2006).
Murray, J. D. et al. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315, 101–104 (2007).
Tirichine, L. et al. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315, 104–107 (2007).
Marsh, J. F. et al. Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol. 144, 324–335 (2007).
Mitra, R. M. & Long, S. R. Plant and bacterial symbiotic mutants define three transcriptionally distinct stages in the development of the Medicago truncatula/Sinorhizobium meliloti symbiosis. Plant Physiol. 134, 595–604 (2004). Plant and rhizobial mutants arrested at different morphologically defined stages of symbiotic development are also arrested in the ability to undergo symbiosis-specific transcriptional changes.
Starker, C. G., Parra-Colmenares, A. L., Smith, L., Mitra, R. M. & Long, S. R. Nitrogen fixation mutants of Medicago truncatula fail to support plant and bacterial symbiotic gene expression. Plant Physiol. 140, 671–680 (2006).
Pislariu, C. I. & Dickstein, R. An IRE-like AGC kinase gene, MtIRE, has unique expression in the invasion zone of developing root nodules in Medicago truncatula. Plant Physiol. 144, 682–694 (2007).
Mergaert, P. et al. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium–legume symbiosis. Proc. Natl Acad. Sci. USA 103, 5230–5235 (2006). Showed that plants that form indeterminate nodules impose a programme of genomic endoreduplication on the bacteria, whereas plants that form determinate nodules do not.
Cebolla, A. et al. The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J. 18, 4476–4484 (1999).
Vinardell, J. M. et al. Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 15, 2093–2105 (2003).
Galitski, T., Saldanha, A. J., Styles, C. A., Lander, E. S. & Fink, G. R. Ploidy regulation of gene expression. Science 285, 251–254 (1999).
Robertson, J. G. & Lyttleton, P. Division of peribacteroid membranes in root nodules of white clover. J. Cell Sci. 69, 147–157 (1984).
Limpens, E. et al. Formation of organelle-like N2-fixing symbiosomes in legume root nodules is controlled by DMI2. Proc. Natl Acad. Sci. USA 102, 10375–10380 (2005). Demonstrated that a Nod factor signalling intermediate DMI2 is required for symbiosome formation as well as early events in Nod factor signalling.
Combier, J. P. et al. MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Genes Dev. 20, 3084–3088 (2006).
Veereshlingam, H. et al. nip, a symbiotic Medicago truncatula mutant that forms root nodules with aberrant infection threads and plant defense-like response. Plant Physiol. 136, 3692–3702 (2004).
Dickstein, R., Scheirer, D. C., Fowle, W. H. & Ausubel, F. M. Nodules elicited by Rhizobium meliloti haem mutants are arrested at an early stage of development. Mol. Gen. Genet. 230, 423–432 (1991).
Gilles-Gonzalez, M. A., Ditta, G. S. & Helinski, D. R. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature 350, 170–172 (1991).
Kereszt, A., Slaska-Kiss, K., Putnoky, P., Banfalvi, Z. & Kondorosi, A. The cycHJKL genes of Rhizobium meliloti involved in cytochrome c biogenesis are required for 'respiratory' nitrate reduction ex planta and for nitrogen fixation during symbiosis. Mol. Gen. Genet. 247, 39–47 (1995).
Catalano, C. M., Lane, W. S. & Sherrier, D. J. Biochemical characterization of symbiosome membrane proteins from Medicago truncatula root nodules. Electrophoresis 25, 519–531 (2004).
Catalano, C. M., Czymmek, K. J., Gann, J. G. & Sherrier, D. J. Medicago truncatula syntaxin SYP132 defines the symbiosome membrane and infection droplet membrane in root nodules. Planta 225, 541–550 (2006). Defined a molecular marker of symbiosome membranes.
Sanderfoot, A. A. & Raikhel, N. V. The specificity of vesicle trafficking: coat proteins and SNAREs. Plant Cell 11, 629–642 (1999).
Mellor, R. B. Bacteroids in the Rhizobium–legume symbiosis inhabit a plant internal lytic compartment: implications for other microbial endosymbioses. J. Exp. Bot. 40, 831–839 (1989).
Roth, L. E. & Stacey, G. Bacterium release into host cells of nitrogen-fixing soybean nodules: the symbiosome membrane comes from three sources. Eur. J. Cell Biol. 49, 13–23 (1989).
Jones, K. M., Lloret, J., Daniele, J. R. & Walker, G. C. The type IV secretion system of Sinorhizobium meliloti strain 1021 is required for conjugation, but not for intracellular symbiosis. J. Bacteriol. 189, 2133–2138 (2006).
Campbell, G. R., Reuhs, B. L. & Walker, G. C. Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core. Proc. Natl Acad. Sci. USA 99, 3938–3943 (2002).
Glazebrook, J., Ichige, A. & Walker, G. C. A Rhizobium meliloti homolog of the Escherichia coli peptide-antibiotic transport protein SbmA is essential for bacteroid development. Genes Dev. 7, 1485–1497 (1993).
Ferguson, G. P. et al. Similarity to peroxisomal-membrane protein family reveals that Sinorhizobium and Brucella BacA affect lipid-A fatty acids. Proc. Natl Acad. Sci. USA 101, 5012–5017 (2004).
Putnoky, P. et al. The pha gene cluster of Rhizobium meliloti involved in pH adaptation and symbiosis encodes a novel type of K+ efflux system. Mol. Microbiol. 28, 1091–1101 (1998).
Mitsui, H., Sato, T., Sato, Y., Ito, N. & Minamisawa, K. Sinorhizobium meliloti RpoH1 is required for effective nitrogen-fixing symbiosis with alfalfa. Mol. Genet. Genom. 271, 416–425 (2004).
Wells, D. H. & Long, S. R. The Sinorhizobium meliloti stringent response affects multiple aspects of symbiosis. Mol. Microbiol. 43, 1115–1127 (2002).
Prell, J. & Poole, P. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 14, 161–168 (2006).
Hunt, S. & Layzell, D. B. Gas exchange of legume nodules and the regulation of nitrogenase activity. Annu. Rev. Plant Physiol. 44, 483–511 (1993).
Jordan, A. & Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 67, 71–98 (1998).
Bardin, S., Dan, S., Osteras, M. & Finan, T. M. A phosphate transport system is required for symbiotic nitrogen fixation by Rhizobium meliloti. J. Bacteriol. 178, 4540–4547 (1996).
Yuan, Z. C., Zaheer, R. & Finan, T. M. Regulation and properties of PstSCAB, a high-affinity, high-velocity phosphate transport system of Sinorhizobium meliloti. J. Bacteriol. 188, 1089–1102 (2006).
Morandi, D., Prado, E., Sagan, M. & Duc, G. Characterisation of new symbiotic Medicago truncatula (Gaertn.) mutants, and phenotypic or genotypic complementary information on previously described mutants. Mycorrhiza 15, 283–289 (2005).
Sherrier, D. J., Borisov, A. Y., Tikhonovich, I. A. & Brewin, N. J. Immunocytological evidence for abnormal symbiosome development in nodules of the pea mutant line Sprint2Fix− (sym31). Protoplasma 199, 57–68 (1997).
Voroshilova, V. A. et al. Effect of mutations in Pisum sativum L. genes blocking different stages of nodule development on the expression of late symbiotic genes in Rhizobium leguminosarum bv. viciae. Mol. Plant Microbe Interact. 14, 471–476 (2001).
Bradley, D. J., Butcher, G. W., Galfre, G., Wood, E. A. & Brewin, N. J. Physical association between the peribacteroid membrane and lipopolysaccharide from the bacteroid outer membrane in Rhizobium-infected pea root nodule cells. J. Cell Sci. 85, 47–61 (1986).
Bolanos, L., Redondo-Nieto, M., Rivilla, R., Brewin, N. J. & Bonilla, I. Cell surface interactions of Rhizobium bacteroids and other bacterial strains with symbiosomal and peribacteroid membrane components from pea nodules. Mol. Plant Microbe Interact. 17, 216–223 (2004).
Fischer, H. M. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 58, 352–386 (1994).
Vasse, J., de Billy, F., Camut, S. & Truchet, G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 172, 4295–4306 (1990). The first observation of a plant defence response associated with failed infection threads.
Yang, W. C., Horvath, B., Hontelez, J., van Kammen, A. & Bisseling, T. In situ localization of Rhizobium mRNAs in pea nodules: nifA and nifH localization. Mol. Plant Microbe Interact. 4, 464–468 (1991).
Poole, P. & Allaway, D. Carbon and nitrogen metabolism in Rhizobium. Adv. Microb. Physiol. 43, 117–163 (2000).
Day, D. A., Poole, P. S., Tyerman, S. D. & Rosendahl, L. Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell. Mol. Life Sci. 58, 61–71 (2001).
Lodwig, E. M. et al. Amino-acid cycling drives nitrogen fixation in the legume–Rhizobium symbiosis. Nature 422, 722–726 (2003).
de Bruijn, F. J. et al. Rhizobium meliloti 1021 has three differentially regulated loci involved in glutamine biosynthesis, none of which is essential for symbiotic nitrogen fixation. J. Bacteriol. 171, 1673–1682 (1989).
Kahn, M. L., McDermott, T. R. & Udvardi, M. K. in The Rhizobiaceae (eds Spaink, H. P., Kondorosi, A. & Hooykaas, P. J. J.) 461–485 (Kluwer Academic Publishers, Dordrecht, 1998).
Barsch, A., Carvalho, H. G., Cullimore, J. V. & Niehaus, K. GC-MS based metabolite profiling implies three interdependent ways of ammonium assimilation in Medicago truncatula root nodules. J. Biotechnol. 127, 79–83 (2006).
Harrison, J., Pou de Crescenzo, M. A., Sene, O. & Hirel, B. Does lowering glutamine synthetase activity in nodules modify nitrogen metabolism and growth of Lotus japonicus? Plant Physiol. 133, 253–262 (2003).
Cordoba, E., Shishkova, S., Vance, C. P. & Hernandez, G. Antisense inhibition of NADH glutamate synthase impairs carbon/nitrogen assimilation in nodules of alfalfa (Medicago sativa L.). Plant J. 33, 1037–1049 (2003).
Willis, L. B. & Walker, G. C. The phbC (poly-β-hydroxybutyrate synthase) gene of Rhizobium (Sinorhizobium) meliloti and characterization of phbC mutants. Can. J. Microbiol. 44, 554–564 (1998).
Povolo, S. et al. Isolation and characterization of mutants of Rhizobium meliloti unable to synthesize poly-β-hydroxybutyrate. Can. J. Microbiol. 40, 823–829 (1994).
Aneja, P., Zachertowska, A. & Charles, T. C. Comparison of the symbiotic and competition phenotypes of Sinorhizobium meliloti PHB synthesis and degradation pathway mutants. Can. J. Microbiol. 51, 599–604 (2005).
Ronson, C. W., Lyttleton, P. & Robertson, J. G. C(4)-dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens. Proc. Natl Acad. Sci. USA 78, 4284–4288 (1981).
Driscoll, B. T. & Finan, T. M. NAD+-dependent malic enzyme of Rhizobium meliloti is required for symbiotic nitrogen fixation. Mol. Microbiol. 7, 865–873 (1993). Showed that rhizobial malic enzyme is required for nitrogen fixation and proposed a model for carbon assimilation by bacteroids.
Ott, T. et al. Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr. Biol. 15, 531–535 (2005). Directly demonstrated that plant leghaemoglobins are required for nitrogen fixation by symbiotic bacteria.
Gordon, A. J., Minchin, F. R., James, C. L. & Komina, O. Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol. 120, 867–878 (1999).
Barratt, D. H. et al. Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol. 127, 655–664 (2001).
Krusell, L. et al. The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 17, 1625–1636 (2005).
Wienkoop, S. & Saalbach, G. Proteome analysis. Novel proteins identified at the peribacteroid membrane from Lotus japonicus root nodules. Plant Physiol. 131, 1080–1090 (2003).
Dos Santos, P. C. et al. Iron–sulfur cluster assembly: NifU-directed activation of the nitrogenase Fe protein. J. Biol. Chem. 279, 19705–19711 (2004).
Timmers, A. C. et al. Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant Microbe Interact. 13, 1204–1213 (2000).
Ausubel, F. M. Are innate immune signaling pathways in plants and animals conserved? Nature Immunol. 6, 973–979 (2005).
Mithofer, A. Suppression of plant defence in rhizobia–legume symbiosis. Trends Plant Sci. 7, 440–444 (2002).
Boller, T. Peptide signalling in plant development and self/non-self perception. Curr. Opin. Cell Biol. 17, 116–122 (2005).
Shibuya, N. & Minami, E. Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant Pathol. 59, 223–233 (2001).
Zeidler, D. et al. Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl Acad. Sci. USA 101, 15811–15816 (2004).
Altschul, S. F., Gish, W., Miller, W., Meyers, E. W. & Lipmann, D. J. A basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Felix, G., Duran, J. D., Volko, S. & Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18, 265–276 (1999).
Scheidle, H., Gross, A. & Niehaus, K. The Lipid A substructure of the Sinorhizobium meliloti lipopolysaccharides is sufficient to suppress the oxidative burst in host plants. New Phytol. 165, 559–565 (2005).
Tellstrom, V. et al. The lipopolysaccharide of Sinorhizobium meliloti suppresses defense-associated gene expression in cell cultures of the host plant Medicago truncatula. Plant Physiol. 143, 825–837 (2007). Showed that the S. meliloti LPS suppresses short-term M. truncatula defence responses such as oxidative burst and longer-term defence responses requiring transcriptional changes.
Angot, A. et al. Ralstonia solanacearum requires F-box-like domain-containing type III effectors to promote disease on several host plants. Proc. Natl Acad. Sci. USA 103, 14620–14625 (2006).
Kaku, H. et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl Acad. Sci. USA 103, 11086–11091 (2006).
van Brussel, A. A., Tak, T., Boot, K. J. & Kijne, J. W. Autoregulation of root nodule formation: signals of both symbiotic partners studied in a split-root system of Vicia sativa subsp. nigra. Mol. Plant Microbe Interact. 15, 341–349 (2002).
Sun, J. et al. Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 46, 961–970 (2006).
Vasse, J., de Billy, F. & Truchet, G. Abortion of infection during the Rhizobium meliloti–alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. Plant J. 4, 555–566 (1993).
Niehaus, K., Kapp, D. & Puhler, A. Plant defence and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPS I)-deficient Rhizobium meliloti mutant. Planta 190, 415–425 (1993).
Tirichine, L., de Billy, F. & Huguet, T. Mtsym6, a gene conditioning Sinorhizobium strain-specific nitrogen fixation in Medicago truncatula. Plant Physiol. 123, 845–851 (2000).
Ivashuta, S. et al. RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell 17, 2911–2921 (2005).
Mithöfer, A., Bhagwat, A. A., Feger, M. & Ebel, J. Suppression of fungal b-glucan-induced plant defence in soybean (Glycine max L.) by cyclic 1, 3-1, 6-β-glucans from the symbiont Bradyrhizobium japonicum. Planta 199, 270–275 (1996).
Bhagwat, A. A. et al. Further studies of the role of cyclic β-glucans in symbiosis. An NdvC mutant of Bradyrhizobium japonicum synthesizes cyclodecakis-(1–3)-β-glucosyl. Plant Physiol. 119, 1057–1064 (1999).
Mithöfer, A., Fliegmann, J., Neuhaus-Url, G., Schwarz, H. & Ebel, J. The hepta-β-glucoside elicitor-binding proteins from legumes represent a putative receptor family. Biol. Chem. 381, 705–713 (2000).
Dow, M., Newman, M. A. & von Roepenack, E. The induction and modulation of plant defense responses by bacterial lipopolysaccharides. Annu. Rev. Phytopathol. 38, 241–261 (2000).
Goldstein, J. et al. Lipopolysaccharide (LPS) from Brucella abortus is less toxic than that from Escherichia coli, suggesting the possible use of B. abortus or LPS from B. abortus as a carrier in vaccines. Infect. Immun. 60, 1385–1389 (1992).
Leigh, J. A., Signer, E. R. & Walker, G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl Acad. Sci. USA 82, 6231–6235 (1985). Demonstrated that succinoglycan is critical for successful infection thread formation.
Aman, P., McNeil, M., Franzen, L. E., Darvill, A. G. & Albersheim, P. Structural elucidation, using HPLC-MS and GLC-MS, of the acidic exopolysaccharide secreted by Rhizobium meliloti strain Rm1021. Carbohydr. Res. 95, 263–282 (1981).
Wang, L. X., Wang, Y., Pellock, B. & Walker, G. C. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 181, 6788–6796 (1999).
Buendia, A. M. et al. The Rhizobium meliloti exoZl exoB fragment of megaplasmid 2: ExoB functions as a UDP-glucose 4-epimerase and ExoZ shows homology to NodX of Rhizobium leguminosarum biovar viciae strain TOM. Mol. Microbiol. 5, 1519–1530 (1991).
Uttaro, A. D., Cangelosi, G. A., Geremia, R. A., Nester, E. W. & Ugalde, R. A. Biochemical characterization of avirulent exoC mutants of Agrobacterium tumefaciens. J. Bacteriol. 172, 1640–1646 (1990).
Reuber, T. L. & Walker, G. C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74, 269–280 (1993). The S. meliloti biosynthetic pathway of succinoglycan was defined by biochemical analysis of exo mutants.
Glucksmann, M. A., Reuber, T. L. & Walker, G. C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J. Bacteriol. 175, 7045–7055 (1993).
Leigh, J. A., Reed, J. W., Hanks, J. F., Hirsch, A. M. & Walker, G. C. Rhizobium meliloti mutants that fail to succinylate their Calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell 51, 579–587 (1987).
Becker, A., Niehaus, K. & Puhler, A. Low-molecular-weight succinoglycan is predominantly produced by Rhizobium meliloti strains carrying a mutated ExoP protein characterized by a periplasmic N-terminal domain and a missing C-terminal domain. Mol. Microbiol. 16, 191–203 (1995).
Imaizumi-Anraku, H. et al. Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature 433, 527–531 (2005).
Kanamori, N. et al. A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc. Natl Acad. Sci. USA 103, 359–364 (2006).
Saito, K. et al. NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 19, 610–624 (2007).
Tirichine, L. et al. Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441, 1153–1156 (2006).
Heckmann, A. B. et al. Lotus japonicus nodulation requires two GRAS-domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol. 142, 1739–1750 (2006).
Schauser, L., Roussis, A., Stiller, J. & Stougaard, J. A plant regulator controlling development of symbiotic root nodules. Nature 402, 191–195 (1999).
LeVier, K., Phillips, R. W., Grippe, V. K., Roop, R. M. & Walker, G. C. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science 287, 2492–2493 (2000). Established that the bacA gene is required for survival of B. abortus within mammalian cells, as well as for survival of S. meliloti within plant cells.
Tsolis, R. M. Comparative genome analysis of the α-proteobacteria: relationships between plant and animal pathogens and host specificity. Proc. Natl Acad. Sci. USA 99, 12503–12505 (2002).
Allen, C. A., Adams, L. G. & Ficht, T. A. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 66, 1008–1016 (1998).
Lestrate, P. et al. Attenuated signature-tagged mutagenesis mutants of Brucella melitensis identified during the acute phase of infection in mice. Infect. Immun. 71, 7053–7060 (2003).
Manterola, L. et al. The lipopolysaccharide of Brucella abortus BvrS/BvrR mutants contains lipid A modifications and has higher affinity for bactericidal cationic peptides. J. Bacteriol. 187, 5631–5639 (2005).
Pelegrini, P. B. & Franco, O. L. Plant γ-thionins: novel insights on the mechanism of action of a multi-functional class of defense proteins. Int. J. Biochem. Cell Biol. 37, 2239–2253 (2005).
Ferguson, G. P., Datta, A., Carlson, R. W. & Walker, G. C. Importance of unusually modified lipid A in Sinorhizobium stress resistance and legume symbiosis. Mol. Microbiol. 56, 68–80 (2005).
Arellano-Reynoso, B. et al. Cyclic β-1, 2-glucan is a Brucella virulence factor required for intracellular survival. Nature Immunol. 6, 618–625 (2005). Demonstrated that B. abortus cyclic-β-glucan is required to prevent endocytic vacuole fusion with the lysosome.
Sola-Landa, A. et al. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29, 125–138 (1998).
Guzman-Verri, C. et al. The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc. Natl Acad. Sci. USA 99, 12375–12380 (2002).
Keating, D. H. Sinorhizobium meliloti SyrA mediates the transcriptional regulation of genes involved in lipopolysaccharide sulfation and exopolysaccharide biosynthesis. J. Bacteriol. 189, 2510–2520 (2007).
Gibson, K. E., Campbell, G. R., Lloret, J. & Walker, G. C. CbrA is a stationary-phase regulator of cell surface physiology and legume symbiosis in Sinorhizobium meliloti. J. Bacteriol. 188, 4508–4521 (2006).
Hallez, R. et al. The asymmetric distribution of the essential histidine kinase PdhS indicates a differentiation event in Brucella abortus. EMBO J. 26, 1444–1455 (2007).
Acknowledgements
We are very grateful to B. K. Minesinger, S. M. Simon, R. V. Woodruff and the reviewers for their helpful comments. We would like to thank members of the Walker laboratory for helpful discussions, and the National Institutes of Health (USA), the Japan Society for the Promotion of Science, the National Science and Research Council of Canada, and the Jane Coffin Childs Fund for Medical Research for funding. G. C. W. is an American Cancer Society Research Professor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
Entrez Protein
FURTHER INFORMATION
Glossary
- Flavonoid
-
A 2-phenyl-1,4-benzopyrone derivative, produced by plants, that serves as a defence and signalling compound.
- Nod factor
-
A lipochitooligosaccharide compound that induces multiple responses that are required for nodulation of appropriate host plants.
- Symbiotic exopolysaccharide
-
A rhizobial secreted β-glucan that is structurally distinct for different species and that mediates infection thread formation.
- Infection thread
-
An ingrowth of the root hair cell membrane, populated with rhizobial bacteria, that progresses inward by new membrane synthesis at the tip.
- Symbiosome
-
A host-derived membrane compartment that originates from the infection thread housing a bacteroid.
- Bacteroid
-
A rhizobial bacterium that has been endocytosed by a plant cell and has elongated and/or branched, and has differentiated or is differentiating into a form that can perform nitrogen fixation.
- Nitrogen fixation
-
The reduction of atmospheric dinitrogen to ammonia.
- Colonized curled root hair
-
A root hair that has been induced by Nod factor to curl around a microcolony of rhizobial bacteria and entrap it.
- Auxin
-
A plant hormone (chiefly indole acetic acid) that regulates plant growth in a concentration-dependent manner.
- Nodule primordium
-
Dedifferentiated, proliferating tissue that develops in the plant cortex during nodule initiation.
- Cyclic β-glucan
-
A cyclized β-1,2 chain of 17–25 glucose residues produced by rhizobial bacteria, Brucella spp. and Agrobacterium spp. that localizes to the periplasm and that functions in osmotolerance and in interaction with host membranes.
- Succinoglycan
-
A symbiotic exopolysaccharide produced by S. meliloti, also known as EPS I, that mediates infection thread formation. An octasaccharide repeating unit modified with acetyl, succinyl and pyruvyl substituents that can be polymerized into a high molecular weight or a low molecular weight form composed of monomers, dimers and trimers.
- Galactoglucan
-
A second exopolysaccharide of S. meliloti (EPS II) that can mediate infection thread formation on M. sativa at a low efficiency and that is produced when an intact copy of the ExpR regulator is present. A disaccharide repeating unit modified with acetyl and pyruvyl substituents.
- Endoreduplication
-
Genomic replication without cytokinesis that results in greater than 2n DNA content within a cell.
- Indeterminate nodule
-
A nodule formed by plants of some clades of legumes that develops a continuously growing nodule meristem at the distal end and has zones of tissue at different stages of development.
- α -proteobacteria
-
A group of bacteria that contains several species able to persist within host-derived membrane-bound compartments in eukaryotic cells. Includes rhizobial bacteria and mammalian pathogens such as Brucella spp., Bartonella spp. and Rickettsia spp.
Rights and permissions
About this article
Cite this article
Jones, K., Kobayashi, H., Davies, B. et al. How rhizobial symbionts invade plants: the Sinorhizobium–Medicago model. Nat Rev Microbiol 5, 619–633 (2007). https://doi.org/10.1038/nrmicro1705
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1705
This article is cited by
-
Exploring the role of symbiotic modifier peptidases in the legume − rhizobium symbiosis
Archives of Microbiology (2024)
-
Targeted mutagenesis of Medicago truncatula Nodule-specific Cysteine-Rich (NCR) genes using the Agrobacterium rhizogenes-mediated CRISPR/Cas9 system
Scientific Reports (2023)
-
Understanding the Interaction and Potential of Halophytes and Associated Microbiome for Bio-saline Agriculture
Journal of Plant Growth Regulation (2023)
-
The microbiome of the endosymbiotic Symbiodiniaceae in corals exposed to thermal stress
Hydrobiologia (2023)
-
Transcriptomic and physiological responses of Rhizobium sp. IRBG74 to Sesbania cannabina and rice (Oryza sativa L) rhizosphere
Plant and Soil (2023)