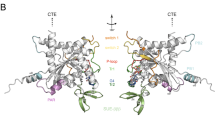
Key Points
-
Septins comprise a conserved family of GTP-binding proteins that have multiple roles during cell division, cytoskeletal organization and membrane remodelling events.
-
Individual septins form smaller core complexes both in vivo and in vitro and contain, depending on the organism, two, three or four septins, each present in two copies. Core complexes oligomerize to form higher-order structures in vivo.
-
Electron microscopy and crystallographic studies have revealed that the septin core structure is mediated by interactions between GTP-binding domains across two distinct dimerization interfaces. Metazoan core complexes, in which the core complex architecture has been determined, oligomerize to form filaments across G-dimer interfaces, whereas the Saccharomyces cerevisiae complex forms complexes across the NC-dimer interface.
-
The G-dimer interface of septin oligomers is similar to the dimerization interface that is observed for the related Toc GTPases, which suggests that mechanistic similarities may exist between these evolutionarily related GTPases. However, the functional consequences of GTP binding and hydrolysis by septins for complex formation remain mysterious.
-
Septin core complexes form higher-order filaments that can dynamically engage the plasma membrane at the bud neck in S. cerevisiae, undergoing complex topological transitions during the cell cycle.
-
It remains unclear how septin-filament assembly is regulated and precisely how GTP binding and hydrolysis, as well as protein cofactors, regulate the assembly and disassembly of septin structure in vivo.
Abstract
Septins comprise a conserved family of proteins that are found primarily in fungi and animals. These GTP-binding proteins have several roles during cell division, cytoskeletal organization and membrane-remodelling events. One factor that is crucial for their functions is the ordered assembly of individual septins into oligomeric core complexes that, in turn, form higher-order structures such as filaments, rings and gauzes. The molecular details of these interactions and the mechanism by which septin-complex assembly is regulated have remained elusive. Recently, the first detailed structural views of the septin core have emerged, and these, along with studies of septin dynamics in vivo, have provided new insight into septin-complex assembly and septin function in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hartwell, L. H. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 69, 265–276 (1971). This study marked the beginning of the septin field with the first identification of septin mutants in S. cerevisiae .
Byers, B. & Goetsch, L. A highly ordered ring of membrane-associated filaments in budding yeast. J. Cell Biol. 69, 717–721 (1976). This classic EM study identified the 10-nm striations at the S. cerevisiae bud neck that would become known as the septin collar.
Haarer, B. K. & Pringle, J. R. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10-nm filaments in the mother–bud neck. Mol. Cell Biol. 7, 3678–3687 (1987).
Kim, H. B., Haarer, B. K. & Pringle, J. R. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J. Cell Biol. 112, 535–544 (1991).
Ford, S. K. & Pringle, J. R. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11 gene product and the timing of events at the budding site. Dev. Genet. 12, 281–292 (1991).
Byers, B. in The Molecular Biology of the Yeast Saccharomyes: Life Cycle and Inheritance (eds Strathern, J. N., Jones, E. W. and Broach, J. R.) 59–96 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1981).
Byers, B. & Goetsch, L. Loss of filamentous ring in cytokinesis-defective mutants of budding yeast. J. Cell Biol. 70, A35 (1976).
Frazier, J. A. et al. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J. Cell Biol. 143, 737–749 (1998).
Field, C. M. et al. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J. Cell Biol. 133, 605–616 (1996).
Kinoshita, M., Field, C. M., Coughlin, M. L., Straight, A. F. & Mitchison, T. J. Self- and actin-templated assembly of mammalian septins. Dev. Cell 3, 791–802 (2002).
Mendoza, M., Hyman, A. A. & Glotzer, M. GTP binding induces filament assembly of a recombinant septin. Curr. Biol. 12, 1858–1863 (2002).
Versele, M. et al. Protein–protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 4568–4583 (2004).
Hall, P. A. & Russell, S. E. The pathobiology of the septin gene family. J. Pathol. 204, 489–505 (2004).
Versele, M. & Thorner, J. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 15, 414–424 (2005).
Pan, F., Malmberg, R. L. & Momany, M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol. Biol. 7, 103 (2007).
Cao, L. et al. Phylogenetic and evolutionary analysis of the septin protein family in metazoan. FEBS Lett. 581, 5526–5532 (2007). This study provides detailed phylogenetic analyses of septins in both fungi and animals. See also reference 15.
Nguyen, T. Q., Sawa, H., Okano, H. & White, J. G. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J. Cell Sci. 113, 3825–3837 (2000).
Kinoshita, M. Assembly of mammalian septins. J. Biochem. 134, 491–496 (2003).
Hall, P. A., Jung, K., Hillan, K. J. & Russell, S. E. Expression profiling the human septin gene family. J. Pathol. 206, 269–278 (2005).
Sirajuddin, M. et al. Structural insight into filament formation by mammalian septins. Nature 449, 311–315 (2007). In this study, the first crystal structure of a septin complex, the human SEPT2–SEPT6–SEPT7 complex, was solved.
Sheffield, P. J. et al. Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J. Biol. Chem. 278, 3483–3488 (2003).
Lukoyanova, N., Baldwin, S. A. & Trinick, J. 3D reconstruction of mammalian septin filaments. J. Mol. Biol. 376, 1–7 (2007).
John, C. M. et al. The Caenorhabditis elegans septin complex is nonpolar. EMBO J. 26, 3296–3307 (2007). This study provides the first evidence that septin complexes are non-polar, using EM to delineate the UNC-59–UNC-61 complex from C. elegans .
Mortensen, E. M., McDonald, H., Yates, J. 3rd & Kellogg, D. R. Cell cycle-dependent assembly of a Gin4–septin complex. Mol. Biol. Cell 13, 2091–2105 (2002).
Vrabioiu, A. M., Gerber, S. A., Gygi, S. P., Field, C. M. & Mitchison, T. J. The majority of the Saccharomyces cerevisiae septin complexes do not exchange guanine nucleotides. J. Biol. Chem. 279, 3111–3118 (2004).
McMurray, M. A. & Thorner, J. in The Septins (eds Hall, P.A., Russell, S. E. G. & Pringle, J. R.) (John Wiley & Sons, Ltd., Chicester, in the press).
Rodal, A. A., Kozubowski, L., Goode, B. L., Drubin, D. G. & Hartwig, J. H. Actin and septin ultrastructures at the budding yeast cell cortex. Mol. Biol. Cell 16, 372–384 (2005). This study provides the first high-resolution views of cortical septin structures in yeast cells using rapid-freeze and deep-etch EM.
Fares, H., Goetsch, L. & Pringle, J. R. Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae. J. Cell Biol. 132, 399–411 (1996).
De Virgilio, C., DeMarini, D. J. & Pringle, J. R. SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiae that is expressed specifically in sporulating cells. Microbiology 142, 2897–2905 (1996).
Ozsarac, N., Bhattacharyya, M., Dawes, I. W. & Clancy, M. J. The SPR3 gene encodes a sporulation-specific homologue of the yeast CDC3/10/11/12 family of bud neck microfilaments and is regulated by ABFI. Gene 164, 157–162 (1995).
Chant, J. Septin scaffolds and cleavage planes in Saccharomyces. Cell 84, 187–190 (1996).
Sanders, S. L. & Herskowitz, I. The BUD4 protein of yeast, required for axial budding, is localized to the mother/BUD neck in a cell cycle-dependent manner. J. Cell Biol. 134, 413–427 (1996).
Halme, A., Michelitch, M., Mitchell, E. L. & Chant, J. Bud10p directs axial cell polarization in budding yeast and resembles a transmembrane receptor. Curr. Biol. 6, 570–579 (1996).
Roemer, T., Madden, K., Chang, J. & Snyder, M. Selection of axial growth sites in yeast requires Axl2p, a novel plasma membrane glycoprotein. Genes Dev. 10, 777–793 (1996).
Kang, P. J., Sanson, A., Lee, B. & Park, H. O. A GDP/GTP exchange factor involved in linking a spatial landmark to cell polarity. Science 292, 1376–1378 (2001).
Kusch, J., Meyer, A., Snyder, M. P. & Barral, Y. Microtubule capture by the cleavage apparatus is required for proper spindle positioning in yeast. Genes Dev. 16, 1627–1639 (2002).
Grava, S., Schaerer, F., Faty, M., Philippsen, P. & Barral, Y. Asymmetric recruitment of dynein to spindle poles and microtubules promotes proper spindle orientation in yeast. Dev. Cell 10, 425–439 (2006).
Barral, Y., Parra, M., Bidlingmaier, S. & Snyder, M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 13, 176–187 (1999).
Longtine, M. S. et al. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell Biol. 20, 4049–4061 (2000).
Longtine, M. S. & Bi, E. Regulation of septin organization and function in yeast. Trends Cell Biol. 13, 403–409 (2003).
Dobbelaere, J. & Barral, Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science 305, 393–396 (2004). This study shows that septin rings act as barriers to compartmentalize the cortex around the site of cytokinesis.
Shulewitz, M. J., Inouye, C. J. & Thorner, J. Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol. Cell Biol. 19, 7123–7137 (1999).
Lew, D. J. The morphogenesis checkpoint: how yeast cells watch their figures. Curr. Opin. Cell Biol. 15, 648–653 (2003).
Keaton, M. A. & Lew, D. J. Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint. Curr. Opin. Microbiol. 9, 540–546 (2006).
Enserink, J. M., Smolka, M. B., Zhou, H. & Kolodner, R. D. Checkpoint proteins control morphogenetic events during DNA replication stress in Saccharomyces cerevisiae. J. Cell Biol. 175, 729–741 (2006).
Smolka, M. B. et al. An FHA domain-mediated protein interaction network of Rad53 reveals its role in polarized cell growth. J. Cell Biol. 175, 743–753 (2006).
Takizawa, P. A., DeRisi, J. L., Wilhelm, J. E. & Vale, R. D. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290, 341–344 (2000). This study demonstrates that a membrane protein specifically translated in the bud is retained in the bud plasma membrane via a septin-dependent diffusion barrier.
Deutschbauer, A. M., Williams, R. M., Chu, A. M. & Davis, R. W. Parallel phenotypic analysis of sporulation and postgermination growth in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 99, 15530–15535 (2002).
Field, C. M. & Kellogg, D. Septins: cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 9, 387–394 (1999).
Carroll, C. W., Altman, R., Schieltz, D., Yates, J. R. & Kellogg, D. The septins are required for the mitosis-specific activation of the Gin4 kinase. J. Cell Biol. 143, 709–717 (1998).
Hanrahan, J. & Snyder, M. Cytoskeletal activation of a checkpoint kinase. Mol. Cell 12, 663–673 (2003).
Barral, Y., Mermall, V., Mooseker, M. S. & Snyder, M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5, 841–851 (2000).
Luedeke, C. et al. Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J. Cell Biol. 169, 897–908 (2005).
Wu, J. Q., Kuhn, J. R., Kovar, D. R. & Pollard, T. D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5, 723–734 (2003).
An, H., Morrell, J. L., Jennings, J. L., Link, A. J. & Gould, K. L. Requirements of fission yeast septins for complex formation, localization, and function. Mol. Biol. Cell 15, 5551–5564 (2004).
Berlin, A., Paoletti, A. & Chang, F. Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 160, 1083–1092 (2003).
Tasto, J. J., Morrell, J. L. & Gould, K. L. An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160, 1093–1103 (2003).
Longtine, M. S. et al. The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8, 106–119 (1996).
Warenda, A. J., Kauffman, S., Sherrill, T. P., Becker, J. M. & Konopka, J. B. Candida albicans septin mutants are defective for invasive growth and virulence. Infect. Immun. 71, 4045–4051 (2003).
Gale, C. et al. Candida albicans Int1p interacts with the septin ring in yeast and hyphal cells. Mol. Biol. Cell 12, 3538–3549 (2001).
Gerami-Nejad, M., Berman, J. & Gale, C. A. Cassettes for PCR-mediated construction of green, yellow, and cyan fluorescent protein fusions in Candida albicans. Yeast 18, 859–864 (2001).
Warenda, A. J. & Konopka, J. B. Septin function in Candida albicans morphogenesis. Mol. Biol. Cell 13, 2732–2746 (2002).
Helfer, H. & Gladfelter, A. S. AgSwe1p regulates mitosis in response to morphogenesis and nutrients in multinucleated Ashbya gossypii cells. Mol. Biol. Cell 17, 4494–4512 (2006).
Westfall, P. J. & Momany, M. Aspergillus nidulans septin AspB plays pre- and postmitotic roles in septum, branch, and conidiophore development. Mol. Biol. Cell 13, 110–118 (2002).
Maddox, A. S., Lewellyn, L., Desai, A. & Oegema, K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev. Cell 12, 827–835 (2007).
Finger, F. P., Kopish, K. R. & White, J. G. A role for septins in cellular and axonal migration in C. elegans. Dev. Biol. 261, 220–234 (2003).
Neufeld, T. P. & Rubin, G. M. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell 77, 371–379 (1994). This study identifies the first septin in metazoans, Pnut, as involved in cytokinesis. It also shows that septin genes, and aspects of their functions, are conserved beyond fungi.
Fares, H., Peifer, M. & Pringle, J. R. Localization and possible functions of Drosophila septins. Mol. Biol. Cell 6, 1843–1859 (1995).
Adam, J. C., Pringle, J. R. & Peifer, M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol. Biol. Cell 11, 3123–3135 (2000).
Hime, G. R., Brill, J. A. & Fuller, M. T. Assembly of ring canals in the male germ line from structural components of the contractile ring. J. Cell Sci. 109, 2779–2788 (1996).
Robinson, D. N. & Cooley, L. Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol. 6, 474–479 (1996).
Spiliotis, E. T., Kinoshita, M. & Nelson, W. J. A mitotic septin scaffold required for mammalian chromosome congression and segregation. Science 307, 1781–1785 (2005).
Spiliotis, E. T. & Nelson, W. J. Here come the septins: novel polymers that coordinate intracellular functions and organization. J. Cell Sci. 119, 4–10 (2006).
Xue, J. et al. Phosphorylation of a new brain-specific septin, G-septin, by cGMP-dependent protein kinase. J. Biol. Chem. 275, 10047–10056 (2000).
Xie, Y. et al. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr. Biol. 17, 1746–1751 (2007).
Tada, T. et al. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr. Biol. 17, 1752–1758 (2007).
Ihara, M. et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell 8, 343–352 (2005).
Kissel, H. et al. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell 8, 353–364 (2005).
Steels, J. D. et al. Sept12 is a component of the mammalian sperm tail annulus. Cell. Motil. Cytoskeleton 64, 794–807 (2007).
Ihara, M. et al. Sept4, a component of presynaptic scaffold and lewy bodies, is required for the suppression of α-synuclein neurotoxicity. Neuron 53, 519–533 (2007).
Ihara, M. et al. Association of the cytoskeletal GTP-binding protein Sept4/H5 with cytoplasmic inclusions found in Parkinson's disease and other synucleinopathies. J. Biol. Chem. 278, 24095–24102 (2003).
Kuhlenbaumer, G. et al. Mutations in SEPT9 cause hereditary neuralgic amyotrophy. Nature Genet. 37, 1044–1046 (2005).
Barral, Y. & Mansuy, I. M. Septins: cellular and functional barriers of neuronal activity. Curr. Biol. 17, R961–963 (2007).
Leipe, D. D., Wolf, Y. I., Koonin, E. V. & Aravind, L. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 317, 41–72 (2002).
Vetter, I. R. & Wittinghofer, A. The guanine nucleotide-binding switch in three dimensions. Science 294, 1299–1304 (2001).
Bos, J. L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (2007).
Sun, Y. J. et al. Crystal structure of pea Toc34, a novel GTPase of the chloroplast protein translocon. Nature Struct. Biol. 9, 95–100 (2002).
Scrima, A. & Wittinghofer, A. Dimerisation-dependent GTPase reaction of MnmE: how potassium acts as GTPase-activating element. EMBO J. 25, 2940–2951 (2006).
Bertin, A. et al. Saccharomyces cerevisiae septins: supramolecular organization of hetero-oligomers and the mechanism of filament assembly. Proc. Natl Acad. Sci. USA in the press. In this study, the architecture of the S. cerevisiae septin core complex was determined using biochemistry and EM.
Versele, M. & Thorner, J. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J. Cell Biol. 164, 701–715 (2004).
Farkasovsky, M., Herter, P., Voss, B. & Wittinghofer, A. Nucleotide binding and filament assembly of recombinant yeast septin complexes. Biol. Chem. 386, 643–656 (2005).
Huang, Y. W., Surka, M. C., Reynaud, D., Pace-Asciak, C. & Trimble, W. S. GTP binding and hydrolysis kinetics of human septin 2. FEBS J. 273, 3248–3260 (2006).
Gasper, R., Scrima, A. & Wittinghofer, A. Structural insights into HypB, a GTP-binding protein that regulates metal binding. J. Biol. Chem. 281, 27492–27502 (2006).
Oreb, M., Tews, I. & Schleiff, E. Policing Tic 'n' Toc, the doorway to chloroplasts. Trends Cell Biol. 18, 19–27 (2008).
Bennett, M. J., Schlunegger, M. P. & Eisenberg, D. 3D domain swapping: a mechanism for oligomer assembly. Protein Sci. 4, 2455–2468 (1995).
Canals, A. et al. The structure of an engineered domain-swapped ribonuclease dimer and its implications for the evolution of proteins toward oligomerization. Structure 9, 967–976 (2001).
Mino, A. et al. Shs1p: a novel member of septin that interacts with spa2p, involved in polarized growth in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 251, 732–736 (1998).
Cid, V. J., Adamikova, L., Sanchez, M., Molina, M. & Nombela, C. Cell cycle control of septin ring dynamics in the budding yeast. Microbiology 147, 1437–1450 (2001).
Zhang, J. et al. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 9, 1458–1467 (1999).
Casamayor, A. & Snyder, M. Molecular dissection of a yeast septin: distinct domains are required for septin interaction, localization, and function. Mol. Cell Biol. 23, 2762–2777 (2003).
Vrabioiu, A. M. & Mitchison, T. J. Symmetry of septin hourglass and ring structures. J. Mol. Biol. 372, 37–49 (2007).
Vrabioiu, A. M. & Mitchison, T. J. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature 443, 466–469 (2006). In references 101 and 102, the authors use polarized fluorescence microscopy to show that septins undergo a rotation during septin ring splitting and confirm that septin filaments lack polarity.
Dobbelaere, J., Gentry, M. S., Hallberg, R. L. & Barral, Y. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev. Cell 4, 345–357 (2003).
Caviston, J. P., Longtine, M., Pringle, J. R. & Bi, E. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14, 4051–4066 (2003).
Longtine, M. S., Fares, H. & Pringle, J. R. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J. Cell Biol. 143, 719–736 (1998).
Asano, S. et al. Direct phosphorylation and activation of a Nim1-related kinase Gin4 by Elm1 in budding yeast. J. Biol. Chem. 281, 27090–27098 (2006).
Weiss, E. L., Bishop, A. C., Shokat, K. M. & Drubin, D. G. Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nature Cell Biol. 2, 677–685 (2000).
Schmidt, M., Varma, A., Drgon, T., Bowers, B. & Cabib, E. Septins, under Cla4p regulation, and the chitin ring are required for neck integrity in budding yeast. Mol. Biol. Cell 14, 2128–2141 (2003).
Kadota, J., Yamamoto, T., Yoshiuchi, S., Bi, E. & Tanaka, K. Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 5329–5345 (2004).
Tang, C. S. & Reed, S. I. Phosphorylation of the septin Cdc3 in G1 by the Cdc28 kinase is essential for efficient septin ring disassembly. Cell Cycle 1, 42–49 (2002).
Johnson, E. S. & Blobel, G. Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J. Cell Biol. 147, 981–994 (1999).
Kinoshita, M. et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 11, 1535–1547 (1997).
Ito, H. et al. Possible role of Rho/Rhotekin signaling in mammalian septin organization. Oncogene 24, 7064–7072 (2005).
Nagata, K. et al. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J. Biol. Chem. 278, 18538–18543 (2003).
Kremer, B. E., Haystead, T. & Macara, I. G. Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol. Biol. Cell 16, 4648–4659 (2005).
Surka, M. C., Tsang, C. W. & Trimble, W. S. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol. Biol. Cell 13, 3532–3545 (2002).
Joberty, G., Perlungher, R. R. & Macara, I. G. The Borgs, a new family of Cdc42 and TC10 GTPase-interacting proteins. Mol. Cell Biol. 19, 6585–6597 (1999).
Joberty, G. et al. Borg proteins control septin organization and are negatively regulated by Cdc42. Nature Cell Biol. 3, 861–866 (2001).
Crooks, G. E., Hon, G., Chandonia, J. M. & Brenner, S. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Gladfelter, A. S., Pringle, J. R. & Lew, D. J. The septin cortex at the yeast mother–bud neck. Curr. Opin. Microbiol. 4, 681–689 (2001).
Acknowledgements
We thank E. Nogales, J. Thorner, M. A. McMurray and colleagues for sharing data before publication and for critical reading of the manuscript. We thank T. L. Weirich for help in computer modelling of septin filaments. We also thank members of the Barral laboratory for discussion. J.P.E. is an European Molecular Biology Organization (EMBO) fellow. C.S.W. is a Human Frontier Science Program (HFSP) fellow.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
41580_2008_BFnrm2407_MOESM1_ESM.pdf
Supplementary information S1 (figure) | Structure-guided sequence alignment of the G-domains of human SEPT2, SEPT6, SEPT7, Arabidopsis thaliana Toc33 and Pisum sativum Toc34. (PDF 1374 kb)
Related links
Related links
DATABASES
Protein Data Bank
FURTHER INFORMATION
Glossary
- Cytokinesis
-
The process of cytoplasmic division.
- Coiled-coil domain
-
A structural motif in proteins that consists of two or more α-helices that twist around each other, similar to a rope, to form a stable, rod-like structure.
- Anaphase
-
The phase during eukaryotic mitosis during which chromosomes are segregated.
- Hyphal growth
-
A type of vegetative growth in fungi in which cells are divided by incomplete septa, allowing transfer of cytoplasm and sometimes of larger structures, such as ribosomes, mitochondria and nuclei, between cells.
- Pseudohyphal growth
-
A type of fungal vegetative growth during which hyphal-like structures are formed. During pseudohyphal growth, cells do not share cytoplasm.
- Cleavage furrow
-
A constriction or indentation of the plasma membrane that marks the beginning of cytokinesis in animal cells.
- Spindle midbody
-
The remnant of the spindle midzone and cleavage furrow in animal cells. The spindle midbody contains proteins and occupies the narrow channel that connects daughter cells upon completion of cytokinesis.
- Cellularization membrane
-
The region of the plasma membrane that encloses nuclei and surrounding cytoplasm to form the cellular blastoderm in Drosophila melanogaster eggs, which contain approximately 5,000 nuclei within a shared cytoplasm.
- P-loop
-
(Phosphate-binding loop). A nucleotide-binding-site motif that is found in many ATPases and GTPases.
- Polarized fluorescence microscopy
-
An optical technique in which polarized light is used to excite a fluorophore. Because excitation depends on the direction of the dipole relative to the polarized light, this method can be used to determine the orientation of a population of fluorophores, provided that they are rigidly and uniformly orientated.
- FRAP
-
A microscopy technique that is used to measure the movement (for example, diffusion rates) of fluorescently tagged molecules over time in vivo. Specific regions of a cell are irreversibly photobleached using a laser; fluorescence is restored by diffusion of fluorescently tagged unbleached molecules into the bleached area.
Rights and permissions
About this article
Cite this article
Weirich, C., Erzberger, J. & Barral, Y. The septin family of GTPases: architecture and dynamics. Nat Rev Mol Cell Biol 9, 478–489 (2008). https://doi.org/10.1038/nrm2407
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2407
This article is cited by
-
Septins, a cytoskeletal protein family, with emerging role in striated muscle
Journal of Muscle Research and Cell Motility (2021)
-
Development of a novel next-generation sequencing panel for diagnosis of quantitative spermatogenic impairment
Journal of Assisted Reproduction and Genetics (2020)
-
Diversity of opisthokont septin proteins reveals structural constraints and conserved motifs
BMC Evolutionary Biology (2019)
-
Cell type-specific CLIP reveals that NOVA regulates cytoskeleton interactions in motoneurons
Genome Biology (2018)
-
Repression of Septin9 and Septin2 suppresses tumor growth of human glioblastoma cells
Cell Death & Disease (2018)