Key Points
-
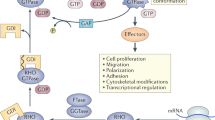
Wiskott–Aldrich Syndrome protein (WASP) is an important regulator of the actin cytoskeleton in haematopoietic cells. WASP-deficiency gives rise to the human disease Wiskott–Aldrich Syndrome (WAS), an X-linked primary immunodeficiency. Constitutively active mutations of WASP have recently been described to give rise to a distinct human disease: X-linked neutropenia.
-
WASP activity is attenuated by multiple signalling pathways downstream of surface receptors. Conformational change, phosphorylation and degradation are important mechanisms.
-
Although WASP does not seem to have a key role in haematopoiesis, WASP confers selective advantage for many mature haematopoietic cell types and is emerging as a key regulator of lymphocyte homeostasis.
-
WASP is required for a diverse range of cell functions in innate and adaptive immune cells. These relate both to the role of WASP in cytoskelatal rearrangement and as an intrinsic signalling molecule.
-
Autoimmunity is an important feature of WAS which is poorly understood. Recent studies suggest that defective regulatory T cell function is an important component.
-
Insights into the basic mechanisms of WASP-associated disease are advancing our understanding of immune regulation with wider application, and this allows the potential for future therapeutic benefit.
Abstract
The Wiskott–Aldrich syndrome protein (WASP) is an important regulator of the actin cytoskeleton that is required for many haematopoietic and immune cell functions, including effective migration, phagocytosis and immune synapse formation. Loss of WASP activity leads to Wiskott–Aldrich syndrome, an X-linked disease that is associated with defects in a broad range of cellular processes, resulting in complex immunodeficiency, autoimmunity and microthrombocytopenia. Intriguingly, gain of function mutations cause a separate disease that is mainly characterized by neutropenia. Here, we describe recent insights into the cellular mechanisms of these two related, but distinct, human diseases and discuss their wider implications for haematopoiesis, immune function and autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Takenawa, T. & Suetsugu, S. The WASP–WAVE protein network: connecting the membrane to the cytoskeleton. Nature Rev. Mol. Cell Biol. 8, 37–48 (2007).
Kurisu, S. & Takenawa, T. The WASP and WAVE family proteins. Genome Biol. 10, 226 (2009).
Symons, M. et al. Wiskott–Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell 84, 723–734 (1996).
Rohatgi, R. et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 (1999).
Machesky, L. M. & Insall, R. H. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347–1356 (1998).
Miki, H. & Takenawa, T. Direct binding of the verprolin-homology domain in N-WASP to actin is essential for cytoskeletal reorganization. Biochem. Biophys. Res. Commun. 243, 73–78 (1998).
Blanchoin, L. et al. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404, 1007–1011 (2000).
Huang, W., Ochs, H. D., Dupont, B. & Vyas, Y. M. The Wiskott–Aldrich syndrome protein regulates nuclear translocation of NFAT2 and NF-κB (RelA) independently of its role in filamentous actin polymerization and actin cytoskeletal rearrangement. J. Immunol. 174, 2602–2611 (2005).
Silvin, C., Belisle, B. & Abo, A. A role for Wiskott–Aldrich syndrome protein in T-cell receptor-mediated transcriptional activation independent of actin polymerization. J. Biol. Chem. 276, 21450–21457 (2001).
Linardopoulou, E. V. et al. Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet. 3, 2477–2485 (2007).
Campellone, K. G., Webb, N. J., Znameroski, E. A. & Welch, M. D. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell 134, 148–161 (2008).
Sullivan, K. E., Mullen, C. A., Blaese, R. M. & Winkelstein, J. A. A multiinstitutional survey of the Wiskott–Aldrich syndrome. J. Pediatr. 125, 876–885 (1994).
Dupuis-Girod, S. et al. Autoimmunity in Wiskott–Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics 111, e622–e627 (2003).
Imai, K. et al. Clinical course of patients with WASP gene mutations. Blood 103, 456–464 (2004).
Devriendt, K. et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nature Genet. 27, 313–317 (2001).
Ancliff, P. J. et al. Two novel activating mutations in the Wiskott–Aldrich syndrome protein result in congenital neutropenia. Blood 108, 2182–2189 (2006).
Beel, K. et al. A large kindred with X-linked neutropenia with an I294T mutation of the Wiskott–Aldrich syndrome gene. Br. J. Haematol. 144, 120–126 (2009).
Kim, A. S., Kakalis, L. T., Abdul-Manan, N., Liu, G. A. & Rosen, M. K. Autoinhibition and activation mechanisms of the Wiskott–Aldrich syndrome protein. Nature 404, 151–158 (2000).
Abdul-Manan, N. et al. Structure of Cdc42 in complex with the GTPase-binding domain of the 'Wiskott–Aldrich syndrome' protein. Nature 399, 379–383 (1999).
Tomasevic, N. et al. Differential regulation of WASP and N-WASP by Cdc42, Rac1, Nck, and PI(4,5)P2. Biochemistry 46, 3494–3502 (2007).
Badour, K. et al. Fyn and PTP-PEST-mediated regulation of Wiskott–Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 199, 99–112 (2004).
Fukuoka, M. et al. A novel neural Wiskott–Aldrich syndrome protein (N-WASP) binding protein, WISH, induces Arp2/3 complex activation independent of Cdc42. J. Cell Biol. 152, 471–482 (2001).
Rohatgi, R., Nollau, P., Ho, H. Y., Kirschner, M. W. & Mayer, B. J. Nck and phosphatidylinositol 4, 5-bisphosphate synergistically activate actin polymerization through the N-WASP–Arp2/3 pathway. J. Biol. Chem. 276, 26448–26452 (2001).
Rivera, G. M., Vasilescu, D., Papayannopoulos, V., Lim, W. A. & Mayer, B. J. A reciprocal interdependence between Nck and PI(4,5)P2 promotes localized N-WASp-mediated actin polymerization in living cells. Mol. Cell 36, 525–535 (2009).
Ho, H. Y. et al. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP–WIP complex. Cell 118, 203–216 (2004).
Padrick, S. B. et al. Hierarchical regulation of WASP/WAVE proteins. Mol. Cell 32, 426–438 (2008).
Cory, G. O., Garg, R., Cramer, R. & Ridley, A. J. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott–Aldrich Syndrome protein. J. Biol. Chem. 277, 45115–45121 (2002).
Park, H. & Cox, D. Cdc42 regulates Fcγ receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott–Aldrich Syndrome protein (WASP) and neural-WASP. Mol. Biol. Cell 20, 4500–1508 (2009).
Dovas, A. et al. Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J. Cell Sci. 122, 3873–3882 (2009).
Cammer, M. et al. The mechanism of CSF-1-induced Wiskott–Aldrich syndrome protein activation in vivo: a role for phosphatidylinositol 3-kinase and Cdc42. J. Biol. Chem. 284, 23302–23311 (2009).
Ma, T., Samanna, V. & Chellaiah, M. A. Dramatic inhibition of osteoclast sealing ring formation and bone resorption in vitro by a WASP-peptide containing pTyr294 amino acid. J. Mol. Signal. 3, 4 (2008).
Blundell, M. P. et al. Phosphorylation of WASp is a key regulator of activity and stability in vivo. Proc. Natl Acad. Sci. USA 106, 15738–15743 (2009).
Torres, E. & Rosen, M. K. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol. Cell 11, 1215–1227 (2003).
Cory, G. O., Cramer, R., Blanchoin, L. & Ridley, A. J. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol. Cell 11, 1229–1239 (2003).
Chou, H. C. et al. WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr. Biol. 16, 2337–2344 (2006).
Konno, A. et al. Differential contribution of Wiskott–Aldrich syndrome protein to selective advantage in T- and B-cell lineages. Blood 103, 676–678 (2004).
de la Fuente, M. A. et al. WIP is a chaperone for Wiskott–Aldrich syndrome protein (WASP). Proc. Natl Acad. Sci. USA 104, 926–931 (2007).
Ramesh, N., Anton, I. M., Hartwig, J. H. & Geha, R. S. WIP, a protein associated with Wiskott–Aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl Acad. Sci. USA 94, 14671–14676 (1997).
Volkman, B. F., Prehoda, K. E., Scott, J. A., Peterson, F. C. & Lim, W. A. Structure of the N-WASP EVH1 domain-WIP complex: insight into the molecular basis of Wiskott–Aldrich Syndrome. Cell 111, 565–576 (2002).
Peterson, F. C. et al. Multiple WASP-interacting protein recognition motifs are required for a functional interaction with N-WASP. J. Biol. Chem. 282, 8446–8453 (2007).
Sasahara, Y. et al. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol. Cell 10, 1269–1281 (2002).
Lim, R. P., Misra, A., Wu, Z. & Thanabalu, T. Analysis of conformational changes in WASP using a split YFP. Biochem. Biophys. Res. Commun. 362, 1085–1089 (2007).
Imai, K., Nonoyama, S. & Ochs, H. D. WASP (Wiskott–Aldrich syndrome protein) gene mutations and phenotype. Curr. Opin. Allergy Clin. Immunol. 3, 427–436 (2003).
Linder, S. et al. Macrophages of patients with X-linked thrombocytopenia display an attenuated Wiskott–Aldrich syndrome phenotype. Immunol. Cell Biol. 81, 130–136 (2003).
Anton, I. M. & Jones, G. E. WIP: A multifunctional protein involved in actin cytoskeleton regulation. Eur. J. Cell Biol. 85, 295–304 (2006).
Anton, I. M. et al. WIP deficiency reveals a differential role for WIP and the actin cytoskeleton in T and B cell activation. Immunity 16, 193–204 (2002).
Parolini, O. et al. Expression of Wiskott–Aldrich syndrome protein (WASP) gene during haematopoietic differentiation. Blood 90, 70–75 (1997).
Wengler, G., Gorlin, J. B., Williamson, J. M., Rosen, F. S. & Bing, D. H. Nonrandom inactivation of the X chromosome in early lineage haematopoietic cells in carriers of Wiskott–Aldrich syndrome. Blood 85, 2471–2477 (1995).
Lacout, C. et al. A defect in haematopoietic stem cell migration explains the nonrandom X-chromosome inactivation in carriers of Wiskott–Aldrich syndrome. Blood 102, 1282–1289 (2003).
Snapper, S. B. et al. Wiskott–Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 9, 81–91 (1998).
Zhang, J. et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott–Aldrich syndrome protein-deficient lymphocytes. J. Exp. Med. 190, 1329–1342 (1999).
Meyer-Bahlburg, A. et al. Wiskott–Aldrich syndrome protein deficiency in B cells results in impaired peripheral homeostasis. Blood 112, 4158–4169 (2008).
Cotta-de-Almeida, V. et al. Wiskott Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proc. Natl Acad. Sci. USA 104, 15424–15429 (2007).
Westerberg, L. S. et al. WASP confers selective advantage for specific haematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function. Blood 112, 4139–4147 (2008).
Davis, B. R. et al. Unprecedented diversity of genotypic revertants in lymphocytes of a patient with Wiskott–Aldrich syndrome. Blood 111, 5064–5067 (2008).
Davis, B. R. & Candotti, F. Revertant somatic mosaicism in the Wiskott–Aldrich syndrome. Immunol. Res. 44, 127–131 (2009).
Ariga, T. et al. Spontaneous in vivo reversion of an inherited mutation in the Wiskott–Aldrich syndrome. J. Immunol. 166, 5245–5249 (2001).
Wada, T. et al. Somatic mosaicism in Wiskott–Aldrich syndrome suggests in vivo reversion by a DNA slippage mechanism. Proc. Natl Acad. Sci. USA 98, 8697–8702 (2001).
Lutskiy, M. I., Sasahara, Y., Kenney, D. M., Rosen, F. S. & Remold-O'Donnell, E. Wiskott–Aldrich syndrome in a female. Blood 100, 2763–2768 (2002).
Wada, T. et al. Second-site mutation in the Wiskott-Aldrich syndrome (WAS) protein gene causes somatic mosaicism in two WAS siblings. J. Clin. Invest. 111, 1389–1397 (2003).
Moulding, D. A. et al. Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J. Exp. Med. 204, 2213–2224 (2007).
Park, J. Y. et al. Early deficit of lymphocytes in Wiskott–Aldrich syndrome: possible role of WASP in human lymphocyte maturation. Clin. Exp. Immunol. 136, 104–110 (2004).
Wada, T., Schurman, S. H., Garabedian, E. K., Yachie, A. & Candotti, F. Analysis of T-cell repertoire diversity in Wiskott–Aldrich syndrome. Blood 106, 3895–3897 (2005).
Gallego, M. D., Santamaria, M., Pena, J. & Molina, I. J. Defective actin reorganization and polymerization of Wiskott–Aldrich T cells in response to CD3-mediated stimulation. Blood 90, 3089–3097 (1997).
Gallego, M. D. et al. WIP and WASP play complementary roles in T cell homing and chemotaxis to SDF-1α. Int. Immunol. 18, 221–232 (2005).
Majstoravich, S. et al. Lymphocyte microvilli are dynamic, actin-dependent structures that do not require Wiskott–Aldrich syndrome protein (WASp) for their morphology. Blood 104, 1396–1403 (2004).
Badour, K. et al. The Wiskott–Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity 18, 141–154 (2003).
Dupre, L. et al. Wiskott–Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity 17, 157–166 (2002).
Cannon, J. L. & Burkhardt, J. K. Differential roles for Wiskott–Aldrich syndrome protein in immune synapse formation and IL-2 production. J. Immunol. 173, 1658–1662 (2004).
Morales-Tirado, V. et al. Cutting edge: selective requirement for the Wiskott–Aldrich syndrome protein in cytokine, but not chemokine, secretion by CD4+ T cells. J. Immunol. 173, 726–730 (2004).
Trifari, S. et al. Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott–Aldrich syndrome patients. J. Immunol. 177, 7451–7461 (2006).
Morales-Tirado, V. et al. Critical requirement for the Wiskott–Aldrich syndrome protein in Th2 effector function. Blood 23 Dec 2009 (doi:10.1182/blood-2009-07-235754).
Zhang, Q. et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361, 2046–2055 (2009).
Engelhardt, K. R. et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J. Allergy Clin. Immunol. 124, 1289–1302 (2009).
Randall, K. L. et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nature Immunol. 10, 1283–1291 (2009).
Westerberg, L. et al. Wiskott–Aldrich syndrome protein deficiency leads to reduced B-cell adhesion, migration, and homing, and a delayed humoral immune response. Blood 105, 1144–1152 (2005).
Kronenberg, M. & Kinjo, Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr. Opin. Immunol. 21, 391–396 (2009).
Berzofsky, J. A. & Terabe, M. The contrasting roles of NKT cells in tumour immunity. Curr. Mol. Med. 9, 667–672 (2009).
Astrakhan, A., Ochs, H. D. & Rawlings, D. J. Wiskott–Aldrich syndrome protein is required for homeostasis and function of invariant NKT cells. J. Immunol. 182, 7370–7380 (2009).
Locci, M. et al. The Wiskott–Aldrich syndrome protein is required for iNKT cell maturation and function. J. Exp. Med. 206, 735–742 (2009).
Lorenzi, R., Brickell, P. M., Katz, D. R., Kinnon, C. & Thrasher, A. J. Wiskott–Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood 95, 2943–2946 (2000).
Leverrier, Y. et al. Cutting edge: the Wiskott–Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J. Immunol. 166, 4831–4834 (2001).
Tsuboi, S. & Meerloo, J. Wiskott–Aldrich syndrome protein is a key regulator of the phagocytic cup formation in macrophages. J. Biol. Chem. 282, 34194–34203 (2007).
Burns, S., Thrasher, A. J., Blundell, M. P., Machesky, L. & Jones, G. E. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood 98, 1142–1149 (2001).
Linder, S., Nelson, D., Weiss, M. & Aepfelbacher, M. Wiskott–Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc. Natl Acad. Sci. USA 96, 9648–9653 (1999).
Zicha, D. et al. Chemotaxis of macrophages is abolished in the Wiskott–Aldrich syndrome. Br. J. Haematol. 101, 659–665 (1998).
Badolato, R. et al. Monocytes from Wiskott–Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J. Immunol. 161, 1026–1033 (1998).
de Noronha, S. et al. Impaired dendritic-cell homing in vivo in the absence of Wiskott–Aldrich syndrome protein. Blood 105, 1590–1597 (2005).
Bouma, G., Burns, S. & Thrasher, A. J. Impaired T-cell priming in vivo resulting from dysfunction of WASp-deficient dendritic cells. Blood 110, 4278–4284 (2007).
Pulecio, J. et al. Expression of Wiskott–Aldrich syndrome protein in dendritic cells regulates synapse formation and activation of naive CD8+ T cells. J. Immunol. 181, 1135–1142 (2008).
Puklin-Faucher, E. & Sheetz, M. P. The mechanical integrin cycle. J. Cell Sci. 122, 179–186 (2009).
Burns, S. et al. Maturation of DC is associated with changes in motile characteristics and adherence. Cell. Motil. Cytoskeleton 57, 118–132 (2004).
Zhang, H. et al. Impaired integrin-dependent function in Wiskott–Aldrich syndrome protein-deficient murine and human neutrophils. Immunity 25, 285–295 (2006).
Orange, J. S. et al. Wiskott–Aldrich syndrome protein is required for NK cell cytotoxicity and co-localizes with actin to NK cell-activating immunologic synapses. Proc. Natl Acad. Sci. USA 99, 11351–11356 (2002).
Gismondi, A. et al. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood 104, 436–443 (2004).
Borg, C. et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 104, 3267–3275 (2004).
Maillard, M. H. et al. The Wiskott–Aldrich syndrome protein is required for the function of CD4+CD25+Foxp3+ regulatory T cells. J. Exp. Med. 204, 381–391 (2007).
Humblet-Baron, S. et al. Wiskott–Aldrich syndrome protein is required for regulatory T cell homeostasis. J. Clin. Invest. 117, 407–418 (2007).
Marangoni, F. et al. WASP regulates suppressor activity of human and murine CD4+CD25+FOXP3+ natural regulatory T cells. J. Exp. Med. 204, 369–380 (2007).
Adriani, M. et al. Impaired in vitro regulatory T cell function associated with Wiskott–Aldrich syndrome. Clin. Immunol. 124, 41–48 (2007).
Ozsahin, H. et al. Long-term outcome following haematopoietic stem-cell transplantation in Wiskott–Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood 111, 439–445 (2008).
Marathe, B. M. et al. Antiplatelet antibodies in WASP− mice correlate with evidence of increased in vivo platelet consumption. Exp. Haematol. 37, 1353–1363 (2009).
Acknowledgements
A.J.T. is a Wellcome Trust Senior Clinical Fellow. S.B. is supported by the Institute of Child Health Biomedical Research Centre and by the primary Immunodeficiency Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Scaffold protein
-
A protein that functions as a support to assemble a multiprotein complex.
- ARP2–ARP3 complex
-
A complex composed of seven proteins, including ARP2, ARP3 and ARP complex protein 1 (ARPC1)–ARPC5. On its own, the complex has little activity but, when bound to an ARP2–ARP3-nucleation-promoting factor, it is activated to generate new actin filaments on pre-existing filaments.
- Actin filaments
-
Formed from the nucleation of monomeric actin subunits during remodelling of the cytoskeleton.
- Thrombocytopenia
-
A lower number of circulating platelets than normal, owing to either the failure of production from bone marrow megakaryocytes or increased clearance from the circulation, predominantly in the spleen.
- Rho family GTPases
-
A subfamily of small GTP-binding proteins that have key roles in rearrangement of the cytoskeleton. The nucleotide-bound state of these GTPases is generally regulated by guanine-nucleotide exchange factors (GEFs), which catalyse GDP–GTP exchange, and GTPase-activating proteins, which facilitate the hydrolysis of the bound GTP. Activation, by extracellular signals through various receptors, results in translocation to the plasma membrane thereby localizing their activity to discrete sites in the cell.
- Calpain
-
One of a group of Ca2+-activated cytoplasmic proteases that are found in many tissues and that hydrolyse various endogenous proteins, including neuropeptides and cytoskeletal proteins, as well as proteins from smooth muscle, cardiac muscle, liver, platelets and erythrocytes. Two subclasses are known: one with high Ca2+ sensitivity and one with low Ca2+ sensitivity.
- Podosome
-
An adhesion structure that is found in various malignant cells and in some normal cells, including macrophages and osteoclasts. Podosomes are small (0.5 μm diameter) structures comprising an actin core surrounded by a ring containing typical focal-adhesion proteins, such as vinculin and paxillin.
- Aorta–gonad–mesonephros region
-
An embryonic site in which the development of definitive haematopoietic stem cells (HSCs) occurs. It comprises the aorta and developing reproductive and excretory (mesonephros) systems. In this haematogenic site, HSCs are concentrated in the aortic region.
- X-chromosome inactivation
-
In females, a single, randomly selected X chromosome is inactivated during early embryogenesis to avoid an imbalance of X-linked genes. This process is controlled by the XIST gene, which produces a large non-protein-encoding RNA that triggers widespread gene silencing on the same X chromosome. If one X chromosome encodes a gene that impairs cell growth or survival, development of cells with the non-silenced normal chromosome is favoured. This is known as apparent non-random X-chromosome inactivation.
- Aneuploidy
-
The occurrence of one or more extra or missing chromosomes, which leads to an unbalanced chromosome complement.
- Immune synapse
-
A discrete contact site classically formed at the point of contact between an antigen-presenting cell (APC) and a T cell. Similar synapses have been described in other immune cells such as natural killer or cytotoxic T cells, where the synapse is formed with a target cell. It is important in establishing cell adhesion and polarity, is influenced by the cytoskeleton and transduces highly controlled secretory signals, thereby allowing the directed release of cytokines or lytic granules towards the APC or target cell.
- Marginal zone
-
A region at the border of the white pulp of the spleen.
- Marginal zone B cell
-
A mature B cell that is enriched in the marginal zone of the spleen. They recognize antigen through semi-invariant receptors, which stimulates their rapid differentiation into antibody-secreting cells. They are thought to be important for host defence against circulating blood-borne pathogens.
- Integrin
-
A member of a large family of transmembrane proteins that traverse the plasma membrane as heterodimers of α- and β-subunits. They have an important role in mediating the interaction of cells with extracellular matrix components, such as fibronectin, and in mediating intracellular cytoskeleton arrangement.
- Invariant NKT (iNKT) cell
-
A lymphocyte that expresses a particular variable gene segment, Vα14 (in mice) and Vα24 (in humans), precisely rearranged to a particular Jα (joining) gene segment to yield T cell receptor α-chains with an invariant sequence. Typically, these cells co-express cell surface markers that are encoded by the natural killer (NK) locus, and they are activated by recognition of CD1d molecules presenting glycolipid antigens.
- Lamellipodia
-
Thin sheet-like processes, which extend at the leading edge of moving cells in an actin-dependent manner, promoted by the Rho family GTPase RAC1.
- Respiratory burst
-
A large increase in oxygen consumption and reactive oxygen species generation that accompanies the exposure of neutrophils to microorganisms and/or inflammatory mediators.
- Eczematous skin disease
-
A clinical process that is associated with severe pathological changes to the skin, which is characterized by redness, oozing, crusting and loss of pigmentation. Histologically, it is characterized by epidermal changes of intracellular oedema, spongiosis or vesiculation.
- Antinuclear antibodies
-
Heterogeneous autoantibodies against one or more antigens present in the nucleus, including chromatin, nucleosomes and ribonuclear proteins. Antinuclear antibodies are found in association with many different autoimmune diseases.
Rights and permissions
About this article
Cite this article
Thrasher, A., Burns, S. WASP: a key immunological multitasker. Nat Rev Immunol 10, 182–192 (2010). https://doi.org/10.1038/nri2724
Issue Date:
DOI: https://doi.org/10.1038/nri2724
This article is cited by
-
Increased cross-presentation by dendritic cells and enhanced anti-tumour therapy using the Arp2/3 inhibitor CK666
British Journal of Cancer (2023)
-
Identification of a novel WAS mutation and the non-splicing effect of a second-site mutation in a Chinese pedigree with Wiskott–Aldrich syndrome
Orphanet Journal of Rare Diseases (2022)
-
Long-term safety and efficacy of lentiviral hematopoietic stem/progenitor cell gene therapy for Wiskott–Aldrich syndrome
Nature Medicine (2022)
-
Utilizing a reductionist model to study host-microbe interactions in intestinal inflammation
Microbiome (2021)
-
Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases
Nature Reviews Rheumatology (2021)