Key Points
-
Human and mouse CD1 proteins are MHC-like molecules that are expressed at the surface of antigen-presenting cells and bind lipids. The recent discovery of many classes of lipid antigens for CD1-restricted T cells implies that the CD1 system surveys cells for changes in their lipid content.
-
Five crystal structures of CD1 proteins bound to lipids have now been solved. These show that the alkyl chains of antigens are inserted into a hydrophobic groove so that their carbohydrate or peptide moieties protrude for contact with T-cell receptors.
-
The general mechanisms of antigen capture by CD1 and MHC proteins differ, as only CD1 uses a deeply buried hydrophobic surface to bind chemically diverse lipid antigens.
-
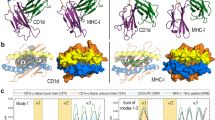
CD1 grooves are composed of up to four antigen-binding pockets, which are known as the A′, C′, F′ and T′ pockets, and up to two antigen-entry portals that are known as the F′ and C′ portals.
-
Glycolipid–CD1a and lipopeptide–CD1a structures show that the A′ pocket is narrow and has a blunt terminus, which allows it to function as a 'molecular ruler' that binds lipids with a defined alkyl chain length.
-
CD1b proteins can bind antigens with a lipid component that is larger or smaller than the antigen-binding groove. This process probably involves chaperone lipids that bind together with small antigens and the ability of long antigens to protrude through portals so that they lie on the outer surface of the protein.
-
Crystal structures show that CD1a, CD1b and CD1d markedly differ in the size, shape and connectivity of their antigen-binding pockets, which implies that each CD1 protein differs in its specificity for lipid ligands.
Abstract
CD1 proteins bind lipids to form antigen complexes that contact T-cell receptors and activate T cells. Recent crystal structures of CD1 proteins show that their antigen-binding grooves are composed of up to four pockets (A′, C′, F′ and T′) and two antigen portals (C′ and F′). Although certain structural features are conserved among CD1 proteins, the grooves of CD1a, CD1b and CD1d differ in the number, shape and connectivity of their antigen-binding pockets. Here, we outline how the portals and pockets of CD1 antigen-binding grooves influence ligand specificity and facilitate the presentation of a surprisingly diverse set of antigenic lipids, glycolipids, lipopeptides and even small, non-lipidic molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Porcelli, S., Morita, C. T. & Brenner, M. B. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature 360, 593–597 (1992). This classic paper provided the first evidence that CD1 proteins can present exogenous antigens to T cells, and it implicated endosomal events in the generation of lipid–CD1 complexes.
Beckman, E. M. et al. Recognition of a lipid antigen by CD1-restricted αβ T cells. Nature 372, 691–694 (1994).
Sieling, P. A. et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269, 227–230 (1995).
Moody, D. B. et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 278, 283–286 (1997). This analysis of T-cell specificity for glycolipid structure showed that CD1b-restricted T cells can precisely discriminate the carbohydrate structures of glycolipid antigens.
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997). This work identified α-galactosylceramides as synthetic antigens that bind CD1d and activate Vα14–Jα281+ T cells (now known as Vα14–Jα18+ T cells). Structure–function analysis showed the role of lipid length and hexose sugars in mediating responses in TCR-transgenic mice, which provides direct evidence for TCR involvement in antigen recognition.
Shamshiev, A. et al. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29, 1667–1675 (1999).
Shamshiev, A. et al. The αβ T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity 13, 255–264 (2000). This functional analysis showed how at least three human CD1 isoforms can present sulphatide antigens to T cells. By highlighting how different CD1 proteins can present the same ligand, these data raise questions about the isoform specificity of antigen recognition.
Moody, D. B. et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404, 884–888 (2000).
Shamshiev, A. et al. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 195, 1013–1021 (2002).
Gilleron, M. et al. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 199, 649–659 (2004).
Fischer, K. et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl Acad. Sci. USA 101, 10685–10690 (2004).
Van Rhijn, I. et al. CD1d-restricted T cell activation by nonlipidic small molecules. Proc. Natl Acad. Sci. USA 101, 13578–13583 (2004).
Moody, D. B. et al. T cell activation by lipopeptide antigens. Science 303, 527–531 (2004).
Matsunaga, I. et al. Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J. Exp. Med. 200, 1559–1569 (2004).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Jackman, R. M. et al. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity 8, 341–351 (1998).
Sugita, M. et al. Separate pathways for antigen presentation by CD1 molecules. Immunity 11, 743–752 (1999).
Chiu, Y. H. et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 189, 103–110 (1999).
De Silva, A. D. et al. Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J. Immunol. 168, 723–733 (2002).
Zeng, Z. et al. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science 277, 339–345 (1997).
Gadola, S. D. et al. Structure of human CD1b with bound ligands at 2.3 Å, a maze for alkyl chains. Nature Immunol. 3, 721–726 (2002). This paper describes the first crystal structure of a CD1 protein bound to lipid ligands, which showed that the carbohydrate and polyalcohol moieties of the GM2 ganglioside and PtdIns protrude from the groove and lie on the TCR contact surface. The complex interconnected nature of the antigen-binding pockets in CD1b resembles a 'maze' for alkyl chains.
Batuwangala, T. et al. The crystal structure of human CD1b with a bound bacterial glycolipid. J. Immunol. 172, 2382–2388 (2003).
Zajonc, D. M. et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity 22, 209–219 (2005).
Zajonc, D. M., Elsliger, M. A., Teyton, L. & Wilson, I. A. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 Å. Nature Immunol. 4, 808–815 (2003). The reported crystal structure of CD1a bound to sulphatide shows that the narrow A′ pocket can bind alkyl chains with a discrete length, so the A′ pocket of CD1a can be thought of as a 'ruler' for alkyl chains.
Joyce, S. et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 279, 1541–1544 (1998).
Gumperz, J. et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12, 211–221 (2000).
Castano, A. R. et al. Peptide binding and presentation by mouse CD1. Science 269, 223–226 (1995).
Moody, D. B. et al. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nature Immunol. 3, 435–442 (2002).
Calabi, F. & Milstein, C. A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature 323, 540–543 (1986). The first report of the human CD1 locus in thymocytes included an analysis that predicted that CD1 proteins would function in the presentation of antigens to T cells.
Angenieux, C. et al. Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J. Biol. Chem. 275, 37757–37764 (2000).
Porcelli, S. et al. Recognition of cluster of differentiation 1 antigens by human CD4−CD8− cytolytic T lymphocytes. Nature 341, 447–450 (1989).
Porcelli, S. A. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59, 1–98 (1995).
Speir, J. A., Stevens, J., Joly, E., Butcher, G. W. & Wilson, I. A. Two different, highly exposed, bulged structures for an unusually long peptide bound to rat MHC class I RT1-Aa. Immunity 14, 81–92 (2001).
Rudensky, A. Y., Preston-Hurlburt, P., al Ramadi, B. K., Rothbard, J. & Janeway, C. A. Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature 359, 429–431 (1992).
Roberts, T. J. et al. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J. Immunol. 168, 5409–5414 (2002).
Prigozy, T. I. et al. Glycolipid antigen processing for presentation by CD1d molecules. Science 291, 664–667 (2001).
Moody, D. B. et al. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J. Exp. Med. 192, 965–976 (2000).
Brossay, L. et al. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188, 1521–1528 (1998).
Sacchettini, J. C. & Gordon, J. I. Rat intestinal fatty acid binding protein. A model system for analyzing the forces that can bind fatty acids to proteins. J. Biol. Chem. 268, 18399–18402 (1993).
Sugita, M. et al. Cytoplasmic tail-dependent localization of CD1b antigen-presenting molecules to MIICs. Science 273, 349–352 (1996).
Ernst, W. A. et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity 8, 331–340 (1998).
Sidobre, S. et al. The Vα14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J. Immunol. 169, 1340–1348 (2002).
Grant, E. P. et al. Molecular recognition of lipid antigens by T cell receptors. J. Exp. Med. 189, 195–205 (1999).
Matsuda, J. L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–754 (2000).
Karadimitris, A. et al. Human CD1d–glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc. Natl Acad. Sci. USA 98, 3294–3298 (2001).
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 (2000).
Melian, A. et al. Molecular recognition of human CD1b antigen complexes: evidence for a common pattern of interaction with αβ TCRs. J. Immunol. 165, 4494–4504 (2000).
Grant, E. P. et al. Fine specificity of TCR complementarity-determining region residues and lipid antigen hydrophilic moieties in the recognition of a CD1–lipid complex. J. Immunol. 168, 3933–3940 (2002).
Ulrichs, T., Moody, D. B., Grant, E., Kaufmann, S. H. & Porcelli, S. A. T-cell responses to CD1-presented lipid antigens in humans with Mycobacterium tuberculosis infection. Infect. Immun. 71, 3076–3087 (2003).
Sugita, M., van Der Wel, N., Rogers, R. A., Peters, P. J. & Brenner, M. B. CD1c molecules broadly survey the endocytic system. Proc. Natl Acad. Sci. USA 97, 8445–8450 (2000).
Jayawardena-Wolf, J., Benlagha, K., Chiu, Y. H., Mehr, R. & Bendelac, A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity 15, 897–908 (2001).
Briken, V., Moody, D. B. & Porcelli, S. A. Diversification of CD1 proteins: sampling the lipid content of different cellular compartments. Semin. Immunol. 12, 517–525 (2000).
Dascher, C. C. & Brenner, M. B. Evolutionary constraints on CD1 structure: insights from comparative genomic analysis. Trends Immunol. 24, 412–418 (2003).
Zeng, G., Wang, X., Robbins, P. F., Rosenberg, S. A. & Wang, R. F. CD4+ T cell recognition of MHC class II-restricted epitopes from NY- ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc. Natl Acad. Sci. USA 98, 3964–3969 (2001). This paper describes the first crystal structure of a CD1 protein that showed how the mouse CD1d heavy chain folds to form a hydrophobic antigen-binding groove with two pockets, A′ and F′, which are analogous to the A and F pockets in MHC class I proteins.
Naidenko, O. et al. Binding and antigen presentation of ceramide-containing glycolipids by soluble mound and human CD1d molecules. J. Exp. Med. 190, 1069–1079 (1999).
Zhou, D. et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science 303, 523–527 (2004).
Kang, S. J. & Cresswell, P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nature Immunol. 5, 175–181 (2004).
Winau, F. et al. Saposin C is required for lipid presentation by human CD1b. Nature Immunol. 5, 169–174 (2004).
Brozovic, S. et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nature Med. 10, 535–539 (2004).
Kraulis, P. J. MOLSCRIPT; a program to produce both detailed and schematic plots of proteins. J. Appl. Cryst. 24, 946–950 (1991).
Merritt, E. A. & Bacon, D. J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Garboczi, D. N. et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384, 134–141 (1996).
Reinherz, E. L. et al. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science 286, 1913–1921 (1999).
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights form the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991).
Rauch, J. et al. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J. Biol. Chem. 278, 47508–47515 (2003).
Wu, D. Y., Segal, N. H., Sidobre, S., Kronenberg, M. & Chapman, P. B. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198, 173–181 (2003).
Jahng, A. et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199, 947–957 (2004).
Brigl, M. & Brenner, M. B. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22, 817–890 (2004).
McDonald, I. K. & Thornton, J. M. Satisfying hydrogen bonding potential in proteins. J. Mol. Biol. 238, 777–793 (1994).
Sheriff, S., Hendrickson, W. A. & Smith, J. L. Structure of myohemerythrin in the azidomet state at 1.7/1.3 Å resolution. J. Mol. Biol. 197, 273–296 (1987).
Acknowledgements
The authors thank C. Roura-Mir, M. Relloso and A. Debono for designs for the CD1 schematics and for discussions and analyses of lipid–CD1 complex structures. This work was supported by grants from the following US organizations: Pew Scholars Program in the Biomedical Sciences, the Cancer Research Institute, The Skaggs Institute for Chemical Biology and the National Institutes of Health (the latter to both D.B.M. and I.A.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ALLELIC POLYMORPHISM
-
Amino-acid sequence alterations in the gene product that are encoded by a given allele, as measured in genetically unrelated members of a population. In contrast to the high rates of allelic polymorphism that produce many MHC molecules with diverse antigen-binding specificities, CD1 proteins show low levels of polymorphism, which are comparable with those of other somatic proteins.
- β-SHEET PLATFORM
-
Several β-strands interact with each other to from a hydrogen-bonded β-sheet that is often referred to as a 'platform' if other structural elements (such as helices) sit atop it, as occurs in CD1 proteins and MHC class I and class II molecules.
- VAN DER WAALS INTERACTIONS
-
Also known as London forces (after Fritz London), these weak intermolecular forces arise from the attractive force between transient dipoles in otherwise non-polar molecules.
- pKa
-
The inverse log of the acid dissociation constant, which can be used to predict the charge state of an amino-acid side chain as a function of pH.
Rights and permissions
About this article
Cite this article
Moody, D., Zajonc, D. & Wilson, I. Anatomy of CD1–lipid antigen complexes. Nat Rev Immunol 5, 387–399 (2005). https://doi.org/10.1038/nri1605
Issue Date:
DOI: https://doi.org/10.1038/nri1605
This article is cited by
-
Phosphatidylinositolmannoside vaccination induces lipid-specific Th1-responses and partially protects guinea pigs from Mycobacterium tuberculosis challenge
Scientific Reports (2023)
-
The role of oxidised self-lipids and alveolar macrophage CD1b expression in COPD
Scientific Reports (2021)
-
Molecular recognition of microbial lipid-based antigens by T cells
Cellular and Molecular Life Sciences (2018)
-
Tailored design of NKT-stimulatory glycolipids for polarization of immune responses
Journal of Biomedical Science (2017)
-
Donor-unrestricted T cells in the human CD1 system
Immunogenetics (2016)