Abstract
The development of obesity and NAFLD is known to be determined by host genetics, diet and lack of exercise. In addition, the gut microbiota has been identified to influence the development of both obesity and NAFLD. Evidence for the role of the gut microbiota has been shown by preclinical studies of transfer of gut microbiota from lean and obese individuals, with the recipient developing the metabolic features of the donor. Many bidirectional interactions of the gut microbiota, including with food, bile and the intestinal epithelium, have been identified. These interactions might contribute to the distinct steps in the progression from lean to obese states, and to steatosis, steatohepatitis and eventually fibrosis. The predominant steps are efficient caloric extraction from the diet, intestinal epithelial damage and greater entry of bacterial components into the portal circulation. These steps result in activation of the innate immune system, liver inflammation and fibrosis. Fortunately, therapeutic interventions might not require a full understanding of these complex interactions. Although antibiotics are too unselective in their action, probiotics have shown efficacy in reversing obesity and NASH in experimental systems, and are under investigation in humans.
Key Points
-
The gastrointestinal tract contains a large and metabolically active microbial community that interacts with the diet and the host
-
The gut microbiota can influence the development of obesity and NAFLD
-
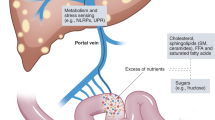
Microbiota characteristics such as efficiency of energy harvest, induction of epithelial damage and entry of microbial components into the portal circulation are probably important in the development of NAFLD
-
Activation of pattern-recognition receptors on hepatic cells by microbiota-derived ligands results in the stimulation of innate immune and profibrotic pathways
-
Therapeutic options under investigation for manipulating the microbiota in NAFLD are prebiotics and probiotics, as well as microbiota transfer
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hattori, M. & Taylor, T. D. The human intestinal microbiome: a new frontier of human biology. DNA Res. 16, 1–12 (2009).
Tremaroli, V. & Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Fry, D. E. Prions: reassessment of the germ theory of disease. J. Am. Coll. Surg. 211, 546–552 (2010).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
Lagier, J. C. et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 18, 1185–1193 (2012).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Weinstock, G. M. Genomic approaches to studying the human microbiota. Nature 489, 250–256 (2012).
Sayasone, S. et al. Diversity of human intestinal helminthiasis in Lao PDR. Trans. R. Soc. Trop. Med. Hyg. 103, 247–254 (2009).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Turnbaugh, P. J. & Gordon, J. I. The core gut microbiome, energy balance and obesity. J. Physiol. 587, 4153–4158 (2009).
Tschop, M. H., Hugenholtz, P. & Karp, C. L. Getting to the core of the gut microbiome. Nat. Biotechnol. 27, 344–346 (2009).
Shade, A. & Handelsman, J. Beyond the Venn diagram: the hunt for a core microbiome. Environ. Microbiol. 14, 4–12 (2012).
Vael, C. & Desager, K. The importance of the development of the intestinal microbiota in infancy. Curr. Opin. Pediatr. 21, 794–800 (2009).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in IL10−/− mice. Nature 487, 104–108 (2012).
Sartor, R. B. Gut microbiota: Diet promotes dysbiosis and colitis in susceptible hosts. Nat. Rev. Gastroenterol. Hepatol. 9, 561–562 (2012).
Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 142, 711–725 (2012).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Zhang, H. et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl Acad. Sci. USA 106, 2365–2370 (2009).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Hildebrandt, M. A. et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137, 1716–1724.e2 (2009).
Turnbaugh, P. J. et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1, 6ra14 (2009).
Murphy, E. F. et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59, 1635–1642 (2010).
Fei, N. & Zhao, L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 7, 880–884 (2013).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 (2012).
Shen, J., Obin, M. S. & Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Aspects Med. 34, 39–58 (2012).
Tarini, J. & Wolever, T. M. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl. Physiol. Nutr. Metab. 35, 9–16 (2010).
Amar, J. et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol. Med. 3, 559–572 (2011).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Backhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Turnbaugh, P. J., Backhed, F., Fulton, L. & Gordon, J. I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 (2008).
Velagapudi, V. R. et al. The gut microbiota modulates host energy and lipid metabolism in mice. J . Lipid Res. 51, 1101–1112 (2010).
Jumpertz, R. et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 94, 58–65 (2011).
Dewulf, E. M. et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 22, 712–722 (2010).
Ge, H. et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149, 4519–4526 (2008).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Figueiredo, R. T., Bittencourt, V. C., Lopes, L. C., Sassaki, G. & Barreto-Bergter, E. Toll-like receptors (TLR2 and TLR4) recognize polysaccharides of Pseudallescheria boydii cell wall. Carbohydr. Res. 356, 260–264 (2012).
Caricilli, A. M. et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 9, e1001212 (2011).
Amar, J. et al. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 87, 1219–1223 (2008).
Ruan, X. et al. Encapsulated Bifidobacteria reduced bacterial translocation in rats following hemorrhagic shock and resuscitation. Nutrition 23, 754–761 (2007).
Brun, P. et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G518–G525 (2007).
Wang, Z. et al. The role of bifidobacteria in gut barrier function after thermal injury in rats. J. Trauma 61, 650–657 (2006).
Sun, L. et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 33, 1925–1932 (2010).
Amar, J. et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia 54, 3055–3061 (2011).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 (2013).
Lichtman, S. N., Keku, J., Schwab, J. H. & Sartor, R. B. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology 100, 513–519 (1991).
Crispe, I. N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Mehal, W. Z. The gut–liver axis: A busy two way street. Hepatology 55, 1647–1649 (2012).
Kubes, P. & Mehal, W. Z. Sterile inflammation in the liver. Gastroenterology 143, 1158–1172 (2012).
Tordjman, J., Guerre-Millo, M. & Clement, K. Adipose tissue inflammation and liver pathology in human obesity. Diabetes Metab. 34, 658–663 (2008).
Aron-Wisnewsky, J., Gaborit, B., Dutour, A. & Clement, K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin. Microbiol. Infect. 19, 338–348 (2013).
Vance, D. E. Role of phosphatidylcholine biosynthesis in the regulation of lipoprotein homeostasis. Curr. Opin. Lipidol. 19, 229–234 (2008).
Zeisel, S. H., Wishnok, J. S. & Blusztajn, J. K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther. 225, 320–324 (1983).
Corbin, K. D. & Zeisel, S. H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 28, 159–165 (2012).
Spencer, M. D. et al. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 140, 976–986 (2011).
Pott, J. & Hornef, M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 13, 684–698 (2012).
Lamkanfi, M. & Dixit, V. M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 28, 137–161 (2012).
Davis, B. K., Wen, H. & Ting, J. P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 (2011).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Lara-Tejero, M. et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203, 1407–1412 (2006).
Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 107, 3076–3080 (2010).
Suzuki, T. et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog . 3, e111 (2007).
Elinav, E., Henao-Mejia, J. & Flavell, R. A. Integrative inflammasome activity in the regulation of intestinal mucosal immune responses. Mucosal Immunol. 6, 4–13 (2013).
Hirota, S. A. et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm. Bowel Dis. 17, 1359–1372 (2011).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Henao-Mejia, J., Elinav, E., Strowig, T. & Flavell, R. A. Inflammasomes: far beyond inflammation. Nat. Immunol. 13, 321–324 (2012).
Miura, K. et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology 139, 323–334 (2010).
Szabo, G., Velayudham, A., Romics, L. Jr & Mandrekar, P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin. Exp. Res. 29, 140S–145S (2005).
Starley, B. Q., Calcagno, C. J. & Harrison, S. A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 51, 1820–1832 (2010).
Stickel, F. & Hellerbrand, C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut 59, 1303–1307 (2010).
Ertle, J. et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer 128, 2436–2443 (2011).
Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516 (2012).
Hernandez-Gea, V., Toffanin, S., Friedman, S. L. & Llovet, J. M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 144, 512–527 (2013).
Irvine, K. M., Schroder, K. & Powell, E. E. Liver repercussions of defective gut surveillance. Hepatology 56, 1174–1177 (2012).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Kirpich, I. A. & McClain, C. J. Probiotics in the treatment of the liver diseases. J. Am. Coll. Nutr. 31, 14–23 (2012).
Cesaro, C. et al. Gut microbiota and probiotics in chronic liver diseases. Dig. Liver Dis. 43, 431–438 (2011).
Frazier, T. H., DiBaise, J. K. & McClain, C. J. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J. Parenter. Enteral. Nutr. 35, 14S–20S (2011).
Li, Z. et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 37, 343–350 (2003).
Ma, X., Hua, J. & Li, Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J. Hepatol. 49, 821–830 (2008).
Velayudham, A. et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology 49, 989–997 (2009).
Nardone, G. et al. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am. J. Physiol. Gastroin test. Liver Physiol. 299, G669–G676 (2010).
Vajro, P. et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Pediatr. Gastroenterol . Nutr. 52, 740–743 (2011).
Aller, R. et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur. Rev. Med . Pharmacol. Sci. 15, 1090–1095 (2011).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Parnell, J. A., Raman, M., Rioux, K. P. & Reimer, R. A. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 32, 701–711 (2011).
Macfarlane, S., Macfarlane, G. T. & Cummings, J. H. Review article: prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 24, 701–714 (2006).
Schwiertz, A. et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18, 190–195 (2010).
Kok, N., Roberfroid, M., Robert, A. & Delzenne, N. Involvement of lipogenesis in the lower VLDL secretion induced by oligofructose in rats. Br. J. Nutr. 76, 881–890 (1996).
Delzenne, N. M. & Williams, C. M. Prebiotics and lipid metabolism. Curr. Opin. Lipidol. 13, 61–67 (2002).
Sugatani, J. et al. Dietary inulin alleviates hepatic steatosis and xenobiotics-induced liver injury in rats fed a high-fat and high-sucrose diet: association with the suppression of hepatic cytochrome P450 and hepatocyte nuclear factor 4alpha expression. Drug. Metab. Dispos. 34, 1677–1687 (2006).
Agheli, N. et al. Plasma lipids and fatty acid synthase activity are regulated by short-chain fructo-oligosaccharides in sucrose-fed insulin-resistant rats. J. Nutr. 128, 1283–1288 (1998).
Sugatani, J. et al. Comparison of enzymatically synthesized inulin, resistant maltodextrin and clofibrate effects on biomarkers of metabolic disease in rats fed a high-fat and high-sucrose (cafeteria) diet. Eur. J. Nutr. 47, 192–200 (2008).
Daubioul, C. et al. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J. Nutr. 132, 967–973 (2002).
Levrat, M. A., Remesy, C. & Demigne, C. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J. Nutr. 121, 1730–1737 (1991).
Topping, D. L. & Clifton, P. M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064 (2001).
Urias-Silvas, J. E. et al. Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp. Br. J . Nutr. 99, 254–261 (2008).
Cani, P. D., Neyrinck, A. M., Maton, N. & Delzenne, N. M. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes. Res. 13, 1000–1007 (2005).
Daubioul, C. A., Horsmans, Y., Lambert, P., Danse, E. & Delzenne, N. M. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: results of a pilot study. Eur. J. Clin. Nutr. 59, 723–726 (2005).
Wickremesekera, K., Miller, G., Naotunne, T. D., Knowles, G. & Stubbs, R. S. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes. Surg. 15, 474–481 (2005).
Mathurin, P. et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 137, 532–540 (2009).
Mai, V., Ukhanova, M., Visone, L., Abuladze, T. & Sulakvelidze, A. Bacteriophage administration reduces the concentration of Listeria monocytogenes in the gastrointestinal tract and its translocation to spleen and liver in experimentally infected mice. Int. J. Microbiol . 2010, 624234 (2010).
Mills, S. et al. Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes 4, 4–16 (2013).
Imaeda, A. B. et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 119, 305–314 (2009).
Hoque, R. et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 141, 358–369 (2011).
Hoque, R. et al. A novel small-molecule enantiomeric analogue of traditional (–)-morphinans has specific TLR9 antagonist properties and reduces sterile inflammation induced organ damage. J. Immunol. 190, 4297–4304 (2013).
Acknowledgements
The work of the author is supported by a VA Merit award.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Mehal, W. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol 10, 637–644 (2013). https://doi.org/10.1038/nrgastro.2013.146
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.146
This article is cited by
-
Mechanisms of Environmental Contributions to Fatty Liver Disease
Current Environmental Health Reports (2019)
-
Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus
Nature Reviews Endocrinology (2018)
-
Nonalcoholic Fatty Liver Disease in Children: Not a Small Matter
Pediatric Drugs (2018)
-
A changing paradigm: management and treatment of the HCV/HIV-co-infected patient
Hepatology International (2018)
-
Mechanistic and therapeutic advances in non-alcoholic fatty liver disease by targeting the gut microbiota
Frontiers of Medicine (2018)