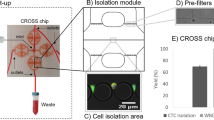
Abstract
The spread of cancer throughout the body is driven by circulating tumour cells (CTCs)1. These cells detach from the primary tumour and move from the bloodstream to a new site of subsequent tumour growth. They also carry information about the primary tumour and have the potential to be valuable biomarkers for disease diagnosis and progression, and for the molecular characterization of certain biological properties of the tumour. However, the limited sensitivity and specificity of current methods for measuring and studying these cells in patient blood samples prevents the realization of their full clinical potential. The use of microfluidic devices is a promising method for isolating CTCs2,3. However, the devices are reliant on three-dimensional structures, which limits further characterization and expansion of cells on the chip. Here we demonstrate an effective approach to isolating CTCs from blood samples of pancreatic, breast and lung cancer patients, by using functionalized graphene oxide nanosheets on a patterned gold surface. CTCs were captured with high sensitivity at a low concentration of target cells (73 ± 32.4% at 3–5 cells per ml blood).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
01 October 2013
In the version of this Letter originally published, in Fig. 1b one of the PEG chains was misplaced. This has now been corrected in the HTML and PDF versions.
References
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New Engl. J Med. 351, 781–791 (2004).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Willipinski-Stapelfeldt, B. et al. Changes in cytoskeletal protein composition indicative of an epithelial–mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin. Cancer Res. 11, 8006–8014 (2005).
Bednarz-Knoll, N., Alix-Panabières, C. & Pantel, K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metastasis Rev. 31, 673–687 (2012).
Pantel, K., Brakenhoff, R. H. & Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature Rev. Cancer 8, 329–340 (2008).
Lin, H. K. et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 16, 5011–5018 (2010).
Dobrovolskaia, M. A. & McNeil, S. E. Immunological properties of engineered nanomaterials. Nature Nanotech. 2, 469–478 (2007).
Xia, X-R., Monteiro-Riviere, N. A. & Riviere, J. E. An index for characterization of nanomaterials in biological systems. Nature Nanotech. 5, 671–675 (2010).
Wang, S. et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew. Chem. Int. Ed. 121, 9132–9135 (2009).
Lee, S-K. et al. Nanowire substrate-based laser scanning cytometry for quantitation of circulating tumor cells. Nano Lett. 12, 2697–2704 (2012).
Zhang, N. et al. Electrospun TiO2 nanofiber-based cell capture assay for detecting circulating tumor cells from colorectal and gastric cancer patients. Adv. Mater. 24, 2756–2760 (2012).
Liu, Z., Robinson, J. T., Sun, X. & Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 130, 10876–10877 (2008).
Mohanty, N. & Berry, V. Graphene-based single-bacterium resolution biodevice and DNA transistor: interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Lett. 8, 4469–4476 (2008).
Ramanathan, T. et al. Functionalized graphene sheets for polymer nanocomposites. Nature Nanotech. 3, 327–331 (2008).
Dreyer, D. R., Park, S., Bielawski, C. W. & Ruoff, R. S. The chemistry of graphene oxide. Chem. Soc. Rev. 39, 228–240 (2010).
Sun, X. et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 1, 203–212 (2008).
Eda, G., Fanchini, G. & Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nature Nanotech. 3, 270–274 (2008).
Li, X. et al. Highly conducting graphene sheets and Langmuir–Blodgett films. Nature Nanotech. 3, 538–542 (2008).
Wang, H., Wang, X., Li, X. & Dai, H. Chemical self-assembly of graphene sheets. Nano Res. 2, 336–342 (2009).
Sieuwerts, A. M. et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J. Natl Cancer Inst. 101, 61–66 (2009).
Salic, A. & Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl Acad. Sci. USA 105, 2415–2420 (2008).
Lustberg, M., Jatana, K. R., Zborowski, M. & Chalmers, J. J. Emerging technologies for CTC detection based on depletion of normal cells. Recent Results Cancer Res. 195, 97–110 (2012).
Stott, S. L. et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl Acad. Sci. USA 107, 18392–18397 (2010).
Mego, M. et al. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int. J. Cancer 129, 417–423 (2011).
Alix-Panabières, C., Schwarzenbach, H. & Pantel, K. Circulating tumor cells and circulating tumor DNA. Annu. Rev. Med. 63, 199–215 (2012).
Ozkumur, E. et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl. Med. 5, 179ra147 (2013).
Krebs, M. G. et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 7, 306–315 (2012).
Edgerton, S. M., Moore, D. II, Merkel, D. & Thor, A. D. erbB-2 (HER-2) and breast cancer progression. Appl. Immunohistochem. Mol. Morphol. 11, 214–221 (2003).
Amir, E. et al. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat. Rev. 38, 708–714 (2012).
Fehm, T. et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res. Treat. 124, 403–412 (2010).
Riethdorf, S. et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin. Cancer Res. 16, 2634–2645 (2010).
Pestrin, M. et al. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent Lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast Cancer Res. Treat. 134, 283–289 (2012).
Acknowledgements
The authors thank K. Herman and S. Laurinec for procurement of patient blood samples, and S. Fouladdel for technical help with qRT-PCR. This work was supported by the National Institutes of Health (NIH) Director's New Innovator Award (1DP2OD006672-01), a Career Development Program of the Gastrointestinal Specialized Program of Research Excellence (GI SPORE) award (CA130810), and a 3M non-tenured Faculty Award. The work was performed in part at the Lurie Nanofabrication Facility, a member of the National Nanotechnology Infrastructure Network, which is supported by the National Science Foundation.
Author information
Authors and Affiliations
Contributions
H.Y. and S.N. conceived and designed the study. H.Y. and T.K. fabricated devices and performed the experiments. T.K., Z.Z. and T.P. performed the cell culture. E.A. analysed the RNA data. C.P., J.L., N.R., M.W., D.H. and D.S. prepared the clinical samples. H.Y. and S.N. analysed the data and co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Information (PDF 15968 kb)
Rights and permissions
About this article
Cite this article
Yoon, H., Kim, T., Zhang, Z. et al. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nature Nanotech 8, 735–741 (2013). https://doi.org/10.1038/nnano.2013.194
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2013.194
This article is cited by
-
Functionalized tetrahedral DNA frameworks for the capture of circulating tumor cells
Nature Protocols (2024)
-
Graphene oxide for photonics, electronics and optoelectronics
Nature Reviews Chemistry (2023)
-
PD-L1-driven efficient enrichment and elimination of circulating cancer cells by magnetic MoSe2 nanosheet
Nano Research (2023)
-
Microfluidics engineering towards personalized oncology—a review
In vitro models (2023)
-
Targeting circulating tumor cells to prevent metastases
Human Cell (2023)