Abstract
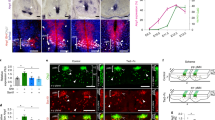
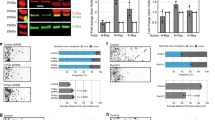
We sought to address the fundamental question of how stem cell microenvironments can regulate self-renewal. We found that Notch was active in astroglia-like neural stem cells (NSCs), but not in transit-amplifying progenitors of the murine subependymal zone, and that the level of Notch transcriptional activity correlated with self-renewal and multipotency. Moreover, dividing NSCs appeared to balance renewal with commitment via controlled segregation of Notch activity, leading to biased expression of known (Hes1) and previously unknown (Egfr) Notch target genes in daughter cells. Pigment epithelium–derived factor (PEDF) enhanced Notch-dependent transcription in cells with low Notch signaling, thereby subverting the output of an asymmetrical division to the production of two highly self-renewing cells. Mechanistically, PEDF induced a non-canonical activation of the NF-κB pathway, leading to the dismissal of the transcriptional co-repressor N-CoR from specific Notch-responsive promoters. Our data provide a basis for stemness regulation in vascular niches and indicate that Notch and PEDF cooperate to regulate self-renewal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Morrison, S.J. & Kimble, J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441, 1068–1074 (2006).
Wu, M. et al. Imaging hematopoietic precursor division in real time. Cell Stem Cell 1, 541–554 (2007).
Riquelme, P.A., Drapeau, E. & Doetsch, F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Phil. Trans. R. Soc. Lond. B 363, 123–137 (2008).
Zhao, C., Deng, W. & Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Palmer, T.D., Willhoite, A.R. & Gage, F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 425, 479–494 (2000).
Shen, Q. et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3, 289–300 (2008).
Tavazoie, M. et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279–288 (2008).
Shen, Q. et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 (2004).
Ramírez-Castillejo, C. et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat. Neurosci. 9, 331–339 (2006).
Tombran-Tink, J. & Barnstable, C.J. PEDF: a multifaceted neurotrophic factor. Nat. Rev. Neurosci. 4, 628–636 (2003).
Notari, L. et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium–derived factor. J. Biol. Chem. 281, 38022–38037 (2006).
Chiba, S. Notch signaling in stem cell systems. Stem Cells 24, 2437–2447 (2006).
Louvi, A. & Artavanis-Tsakonas, S. Notch signaling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93–102 (2006).
Bray, S.J. Notch signaling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Bio. 7, 678–689 (2006).
Alexson, T.O., Hitoshi, S., Coles, B.L., Bernstein, A. & van der Kooy, D. Notch signaling is required to maintain all neural stem cell populations—irrespective of spatial or temporal niche. Dev. Neurosci. 28, 34–48 (2006).
Androutsellis-Theotokis, A. et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442, 823–826 (2006).
Hitoshi, S. et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 16, 846–858 (2002).
Ohtsuka, T., Sakamoto, M., Guillemot, F. & Kageyama, R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J. Biol. Chem. 276, 30467–30474 (2001).
Duncan, A.W. et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6, 314–322 (2005).
Mizutani, K., Yoon, K., Dang, L., Tokunaga, A. & Gaiano, N. Differential Notch signaling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 (2007).
Breunig, J.J., Silbereis, J., Vaccarino, F.M., Sestan, N. & Rakic, P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc. Natl. Acad. Sci. USA 104, 20558–20563 (2007).
Jarriault, S. et al. Signaling downstream of activated mammalian Notch. Nature 377, 355–358 (1995).
Campos, L.S., Decker, L., Taylor, V. & Skarnes, W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J. Biol. Chem. 281, 5300–5309 (2006).
Mori, T. et al. Inducible gene deletion in astroglia and radial glia—a valuable tool for functional and lineage analysis. Glia 54, 21–34 (2006).
Sun, Y., Goderie, S.K. & Temple, S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron 45, 873–886 (2005).
Zhang, Y.W. et al. Presenilin/gamma-secretase–dependent processing of beta-amyloid precursor protein regulates EGF receptor expression. Proc. Natl. Acad. Sci. USA 104, 10613–10618 (2007).
Hermanson, O., Jepsen, K. & Rosenfeld, M.G. N-CoR controls differentiation of neural stem cells into astrocytes. Nature 419, 934–939 (2002).
Kao, H.Y. et al. A histone deacetylase co-repressor complex regulates the Notch signal transduction pathway. Genes Dev. 12, 2269–2277 (1998).
Rosenfeld, M.G., Lunyak, V.V. & Glass, C.K. Sensors and signals: a coactivator/co-repressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 20, 1405–1428 (2006).
Reizis, B. & Leder, P. Direct induction of T lymphocyte–specific gene expression by the mammalian Notch signaling pathway. Genes Dev. 16, 295–300 (2002).
Yabe, T., Sanagi, T., Schwartz, J.P. & Yamada, H. Pigment epithelium–derived factor induces pro-inflammatory genes in neonatal astrocytes through activation of NF-kappaB and CREB. Glia 50, 223–234 (2005).
Yabe, T., Wilson, D. & Schwartz, J.P. NFkappaB activation is required for the neuroprotective effects of pigment epithelium–derived factor (PEDF) on cerebellar granule neurons. J. Biol. Chem. 276, 43313–43319 (2001).
Denis-Donini, S., Caprini, A., Frassoni, C. & Grilli, M. Members of the NF-kappaB family expressed in zones of active neurogenesis in the postnatal and adult mouse brain. Brain Res. Dev. Brain Res. 154, 81–89 (2005).
Chen, L.F. & Greene, W.C. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Bio. 5, 392–401 (2004).
DiDonato, J. et al. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 16, 1295–1304 (1996).
Harhaj, E.W. & Sun, S.C. Regulation of RelA subcellular localization by a putative nuclear export signal and p50. Mol. Cell. Biol. 19, 7088–7095 (1999).
Carlén, M. et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 12, 259–267 (2009).
Cai, J., Jiang, W.G., Grant, M.B. & Boulton, M. Pigment epithelium–derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J. Biol. Chem. 281, 3604–3613 (2006).
Doetsch, F., Petreanu, L., Caille, I., Garcia-Verdugo, J.M. & Alvarez-Buylla, A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36, 1021–1034 (2002).
Ciccolini, F., Mandl, C., Holzl-Wenig, G., Kehlenbach, A. & Hellwig, A. Prospective isolation of late development multipotent precursors whose migration is promoted by EGFR. Dev. Biol. 284, 112–125 (2005).
Yoon, K. & Gaiano, N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 8, 709–715 (2005).
Sang, L., Coller, H.A. & Roberts, J.M. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science 321, 1095–1100 (2008).
Chung, C. et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL). J. Hepatol. 48, 471–478 (2008).
Ang, H.L. & Tergaonkar, V. Notch and NFkappaB Signaling pathways: do they collaborate in normal vertebrate brain development and function? Bioessays 29, 1039–1047 (2007).
Espinosa, L., Santos, S., Ingles-Esteve, J., Munoz-Canoves, P. & Bigas, A. p65-NFkappaB synergizes with Notch to activate transcription by triggering cytoplasmic translocation of the nuclear receptor corepressor N-CoR. J. Cell Sci. 115, 1295–1303 (2002).
Givogri, M.I. et al. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev. Neurosci. 28, 81–91 (2006).
Hsieh, J. & Gage, F.H. Chromatin remodeling in neural development and plasticity. Curr. Opin. Cell Biol. 17, 664–671 (2005).
Baek, S.H. et al. Exchange of N-CoR co-repressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell 110, 55–67 (2002).
Ferrón, S.R. et al. A combined ex/in vivo assay to detect effects of exogenously added factors in neural stem cells. Nat. Protoc. 2, 849–859 (2007).
Acknowledgements
We are grateful to A. Bigas, J.L. de la Pompa, N. Gaiano, W.C. Greene, O. Hermanson, S. Hitoshi, A. Israel, K. Jepsen, M. Karin, R. Kopan, G. Leclercq, M.G. Rosenfeld, S. Sun, D. van der Kooy, Y. Zhan and V. Meni for kindly providing constructs and reagents. We also thank E. Porlan and H. Mira for technical advice, M.P. Rubio for excellent technical assistance, and E. Porlan and S.R. Ferrón for critical reading of the manuscript and valuable discussions. We gratefully acknowledge the help of M.A. Marqués-Torrejón with the infusion experiments and of A. Martínez and D. Gil with cytometry. We are also grateful to E. Lai for comments on the manuscript. This work was supported by grants from Ministerio de Ciencia e Innovación (SAF Program), Ministerio de Sanidad (Red Tercel, Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas, CIBERNED), Generalitat Valenciana (Programa Prometeo) and Fundación 'la Caixa'. C.A.-A. was a recipient of a Formación del Profesorado Universitario predoctoral fellowship. J.M.M.-R. and A.C.D. were funded by CIBERNED.
Author information
Authors and Affiliations
Contributions
All of the authors designed and discussed the experiments. C.A.-A. conducted most of the cell culture and biochemistry experiments on the relationship between PEDF and Notch, Notch/EGFR asymmetry and the role of N-CoR and p65, as well as the infusion experiments and in vivo analyses. C.A.-A. and J.M.M.-R. performed the FACS experiments and analyses with TNR cells, ChIP studies and luciferase assays. A.C.D. contributed to immunohistochemistry analyses and multipotency assays. I.F. supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have an active patent on the use of PEDF for stem-cell renewal.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 3237 kb)
Rights and permissions
About this article
Cite this article
Andreu-Agulló, C., Morante-Redolat, J., Delgado, A. et al. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci 12, 1514–1523 (2009). https://doi.org/10.1038/nn.2437
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2437
This article is cited by
-
Angiocrine endothelium: from physiology to cancer
Journal of Translational Medicine (2020)
-
Adult Neurogenesis in the Subventricular Zone and Its Regulation After Ischemic Stroke: Implications for Therapeutic Approaches
Translational Stroke Research (2020)
-
Dynamic Notch signalling regulates neural stem cell state progression in the Drosophila optic lobe
Neural Development (2018)
-
The effects of pigment epithelium-derived factor on atherosclerosis: putative mechanisms of the process
Lipids in Health and Disease (2018)
-
FACS isolation of endothelial cells and pericytes from mouse brain microregions
Nature Protocols (2018)