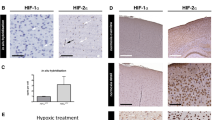
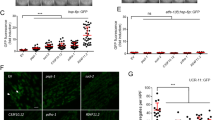
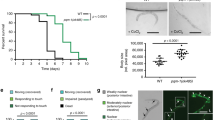
Abstract
Oxygen deprivation can cause severe defects in human brain development, yet the precise cellular and molecular consequences of varying oxygen levels on nervous system development are unknown. We found that hypoxia caused specific axon pathfinding and neuronal migration defects in C. elegans that result from the stabilization of the transcription factor HIF-1 (hypoxia-inducible factor 1) in neurons and muscle. Stabilization of HIF-1 through removal of the proteasomal HIF-1 degradatory pathway phenocopies the hypoxia-induced neuronal defects. Hypoxia-mediated defects in nervous system development depended on signaling through the insulin-like receptor DAF-2, which serves to control the level of reactive oxygen species that also affects axon pathfinding. Hypoxia exerted its effect on axon pathfinding, at least in part, through HIF-1–dependent regulation of the Eph receptor VAB-1. HIF-1–mediated upregulation of VAB-1 protected embryos from hypoxia-induced lethality, but increased VAB-1 levels elicited aberrant axon pathfinding. Similar genetic pathways may cause aberrant human brain development under hypoxic conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arpino, C. et al. Brain damage in preterm infants: etiological pathways. Ann. Ist. Super. Sanita 41, 229–237 (2005).
Inder, T.E. & Volpe, J.J. Mechanisms of perinatal brain injury. Semin. Neonatol. 5, 3–16 (2000).
Berger, R. & Garnier, Y. Pathophysiology of perinatal brain damage. Brain Res. Brain Res. Rev. 30, 107–134 (1999).
El-Khodor, B.F. & Boksa, P. Transient birth hypoxia increases behavioral responses to repeated stress in the adult rat. Behav. Brain Res. 107, 171–175 (2000).
Semenza, G.L. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 7, 345–350 (2001).
Ratcliffe, P.J., Pugh, C.W. & Maxwell, P.H. Targeting tumors through the HIF system. Nat. Med. 6, 1315–1316 (2000).
Iyer, N.V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12, 149–162 (1998).
Shen, C., Nettleton, D., Jiang, M., Kim, S.K. & Powell-Coffman, J.A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 280, 20580–20588 (2005).
Bishop, T. et al. Genetic analysis of pathways regulated by the von Hippel-Lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol. 2, e289 (2004).
Shen, C. & Powell-Coffman, J.A. Genetic analysis of hypoxia signaling and response in C elegans. Ann. NY Acad. Sci. 995, 191–199 (2003).
Epstein, A.C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Jiang, H., Guo, R. & Powell-Coffman, J.A. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl. Acad. Sci. USA 98, 7916–7921 (2001).
Boulin, T., Pocock, R. & Hobert, O. A novel Eph receptor-interacting IgSF protein provides C. elegans motoneurons with midline guidepost function. Curr. Biol. 16, 1871–1883 (2006).
Brunelle, J.K. et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1, 409–414 (2005).
Chandel, N.S. et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 95, 11715–11720 (1998).
Jensen, L.T. & Culotta, V.C. Activation of CuZn superoxide dismutases from Caenorhabditis elegans does not require the copper chaperone CCS. J. Biol. Chem. 280, 41373–41379 (2005).
Vanfleteren, J.R. & De Vreese, A. The gerontogenes age-1 and daf-2 determine metabolic rate potential in aging Caenorhabditis elegans. FASEB J. 9, 1355–1361 (1995).
Honda, Y. & Honda, S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 13, 1385–1393 (1999).
Murphy, C.T. et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283 (2003).
Panowski, S.H., Wolff, S., Aguilaniu, H., Durieux, J. & Dillin, A. PHA-4/Foxa mediates diet-restriction–induced longevity of C. elegans. Nature 447, 550–555 (2007).
Scott, B.A., Avidan, M.S. & Crowder, C.M. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296, 2388–2391 (2002).
George, S.E., Simokat, K., Hardin, J. & Chisholm, A.D. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell 92, 633–643 (1998).
Murai, K.K. & Pasquale, E.B. 'Eph'ective signaling: forward, reverse and crosstalk. J. Cell Sci. 116, 2823–2832 (2003).
Bülow, H.E., Boulin, T. & Hobert, O. Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron 42, 367–374 (2004).
Hobert, O. & Bülow, H. Development and maintenance of neuronal architecture at the ventral midline of C. elegans. Curr. Opin. Neurobiol. 13, 70–78 (2003).
Vihanto, M.M. et al. Hypoxia upregulates expression of Eph receptors and ephrins in mouse skin. FASEB J. 19, 1689–1691 (2005).
O'Driscoll, C.M. & Gorman, A.M. Hypoxia induces neurite outgrowth in PC12 cells that is mediated through adenosine A2A receptors. Neuroscience 131, 321–329 (2005).
Simmer, F. et al. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 1, E12 (2003).
Acknowledgements
We thank Q. Chen for expert technical assistance in generating transgenic strains, A. Clarke for technical help, the Caenorhabditis Genetics Center and J.A. Powell-Coffman for providing strains, M. Krause for the unc-120 promoter, the C. elegans knockout consortia lead by R. Barstead, D. Moerman and S. Mitani for gk, ok and tm alleles, respectively, T. Boulin for the pF25B3.3::vab-1 construct and communicating results on lin-18, and I. Greenwald and members of the Hobert lab for comments on the manuscript. This work was funded in part by the Muscle Dystrophy Association (O.H.). O.H. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
R.P. designed and conducted the experiments and wrote the manuscript. O.H. supervised the project, gave suggestions and helped write the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2, Supplementary Tables 1 and 2, and Supplementary Methods (PDF 475 kb)
Rights and permissions
About this article
Cite this article
Pocock, R., Hobert, O. Oxygen levels affect axon guidance and neuronal migration in Caenorhabditis elegans. Nat Neurosci 11, 894–900 (2008). https://doi.org/10.1038/nn.2152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2152
This article is cited by
-
The hypoxia response pathway promotes PEP carboxykinase and gluconeogenesis in C. elegans
Nature Communications (2022)
-
Sevoflurane Postconditioning Ameliorates Neuronal Migration Disorder Through Reelin/Dab1 and Improves Long-term Cognition in Neonatal Rats After Hypoxic-Ischemic Injury
Neurotoxicity Research (2021)
-
Temporal Dysynchrony in brain connectivity gene expression following hypoxia
BMC Genomics (2016)
-
Axotomy-induced HIF-serotonin signalling axis promotes axon regeneration in C. elegans
Nature Communications (2016)
-
Prolyl hydroxylase regulates axonal rewiring and motor recovery after traumatic brain injury
Cell Death & Disease (2015)