Abstract
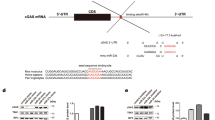
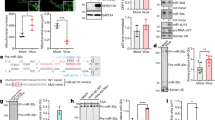
MicroRNAs are small, non-coding RNAs that negatively regulate gene expression1. It has been proposed that microRNAs could function in the regulation of innate immunity2,3, but this has not been demonstrated for viral infection. Here we test this hypothesis using the human pathogenic virus Kaposi's sarcoma-associated herpesvirus (KSHV) and one of its putative natural cellular targets, primary lymphatic endothelial cells4 (LECs). We show that an early antiviral microRNA response (6 h post-infection) includes expression of microRNAs that enhance viral gene expression. In particular, the CREB-induced miR-132 microRNA is highly upregulated after infection and has a negative effect on the expression of interferon-stimulated genes, facilitating viral replication. We show a similar function for miR-132 during infection of monocytes with herpes simplex virus-1 (HSV-1) and human cytomegalovirus (HCMV). miR-132 regulates innate antiviral immunity by inhibiting expression of the p300 transcriptional co-activator. p300 is downregulated early after KSHV infection, and inhibition of miR-132 induction restores p300 expression. Furthermore, p300 regulates miR-132 levels, revealing a dynamic equilibrium between miR-132 and p300. By targeting p300, rather than a transcription factor or signalling protein, miR-132 has a broad role in the regulation of antiviral immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Baltimore, D., Boldin, M. P., O'Connell, R. M., Rao, D. S. & Taganov, K. D. MicroRNAs: new regulators of immune cell development and function. Nat. Immunol. 9, 839–845 (2008).
Lodish, H. F., Zhou, B., Liu, G. & Chen, C. Z. Micromanagement of the immune system by microRNAs. Nat. Rev. Immunol. 8, 120–130 (2008).
Wang, H. W. et al. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36, 687–693 (2004).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Liew, F. Y., Xu, D., Brint, E. K. & O'Neill, L. A. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 (2005).
Taganov, K. D., Boldin, M. P., Chang, K. J. & Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl Acad. Sci. USA 103, 12481–12486 (2006).
Ceppi, M. et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl Acad. Sci. USA 106, 2735–2740 (2009).
Boshoff, C. & Weiss, R. AIDS-related malignancies. Nat. Rev. Cancer 2, 373–382 (2002).
Pfeffer, S. et al. Identification of microRNAs of the herpesvirus family. Nat. Methods 2, 269–276 (2005).
Cai, X. et al. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl Acad. Sci. USA 102, 5570–5575 (2005).
Lagos, D. et al. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe 4, 470–483 (2008).
Cheng, H. Y. et al. microRNA modulation of circadian-clock period and entrainment. Neuron 54, 813–829 (2007).
Klein, M. E. et al. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat. Neurosci. 10, 1513–1514 (2007).
Vo, N. et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl Acad. Sci. USA 102, 16426–16431 (2005).
Wayman, G. A. et al. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc. Natl Acad. Sci. USA 105, 9093–9098 (2008).
Wu, J. & Xie, X. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol. 7, R85 (2006).
Calin, G. A. et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc. Natl Acad. Sci. USA 101, 11755–11760 (2004).
Sarasin-Filipowicz, M., Krol, J., Markiewicz, I., Heim, M. H. & Filipowicz, W. Decreased levels of microRNA miR-122 in individuals with hepatitis C responding poorly to interferon therapy. Nat. Med. 15, 31–33 (2009).
Mayr, B. & Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2, 599–609 (2001).
Xing, J., Ginty, D. D. & Greenberg, M. E. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273, 959–963 (1996).
Xing, J., Kornhauser, J. M., Xia, Z., Thiele, E. A. & Greenberg, M. E. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell Biol. 18, 1946–1955 (1998).
Sadagopan, S. et al. Kaposi's sarcoma-associated herpesvirus induces sustained NF-kappaB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J. Virol. 81, 3949–3968 (2007).
Davies, S. P., Reddy, H., Caivano, M. & Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 (2000).
Gonzalez, G. A. & Montminy, M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59, 675–680 (1989).
Kertesz, M., Iovino, N., Unnerstall, U., Gaul, U. & Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 39, 1278–1284 (2007).
Grimson, A. et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105 (2007).
Merika, M., Williams, A. J., Chen, G., Collins, T. & Thanos, D. Recruitment of CBP/p300 by the IFN-β enhanceosome is required for synergistic activation of transcription. Mol. Cell 1, 277–287 (1998).
Horwitz, G. A. et al. Adenovirus small e1a alters global patterns of histone modification. Science 321, 1084–1085 (2008).
Pauley, K. M. et al. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res. Ther. 10, R101 (2008).
Li, M. et al. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor. Mol. Cell Biol. 20, 8254–8263 (2000).
Lin, R. et al. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20, 800–811 (2001).
Goodman, R. H. & Smolik, S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14, 1553–1577 (2000).
Umbach, J. L. & Cullen, B. R. The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes Dev. 23, 1151–1164 (2009).
Iyer, N. G., Ozdag, H. & Caldas, C. p300/CBP and cancer. Oncogene 23, 4225–4231 (2004).
Marmorstein, R. & Trievel, R. C. Histone modifying enzymes: structures, mechanisms, and specificities. Biochim. Biophys. Acta 1789, 58–68 (2009).
Bourboulia, D. et al. Short- and long-term effects of highly active antiretroviral therapy on Kaposi sarcoma-associated herpesvirus immune responses and viraemia. AIDS 18, 485–493 (2004).
Fabani, M. M. & Gait, M. J. miR-122 targeting with LNA/2'-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. RNA 14, 336–346 (2008).
Smyth, G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article 3 (2004).
Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N. & Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284 (2001).
Acknowledgements
This work was funded by the U.K. Medical Research Council and Cancer Research U.K. We thank G. Wilkinson and R. Stanton for providing HCMV and C. Atkinson for help with HCMV qPCR. We are grateful to members of the Cancer Research UK Viral Oncology Group, in particular J. Funes and V. Emuss for advice and discussions.
Author information
Authors and Affiliations
Contributions
D.L. and C.B. designed experiments and wrote the paper. D.L., G.P. and M.F. performed experiments. S.H. performed bioinformatic analyses. F.Gr. constructed reagents. R.M. provided HCMV and HSV-1 and contributed to experimental design. F.Go. contributed to experimental design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 891 kb)
Supplementary Information
Supplementary Information (XLS 202 kb)
Rights and permissions
About this article
Cite this article
Lagos, D., Pollara, G., Henderson, S. et al. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol 12, 513–519 (2010). https://doi.org/10.1038/ncb2054
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2054
This article is cited by
-
MiRNA-132 regulates the development of osteoarthritis in correlation with the modulation of PTEN/PI3K/AKT signaling
BMC Geriatrics (2021)
-
Relationship between circulating miR-132 and non-alcoholic fatty liver disease in a Chinese population
Hereditas (2020)
-
MicroRNA-132 regulates salt-dependent steady-state renin levels in mice
Communications Biology (2020)
-
A role for miR-132 in learned safety
Scientific Reports (2019)
-
Role of miR-9-5p in preventing peripheral neuropathy in patients with rheumatoid arthritis by targeting REST/miR-132 pathway
In Vitro Cellular & Developmental Biology - Animal (2019)