Abstract
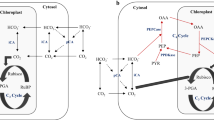
Nearly 50 years ago, inorganic carbon was shown to be fixed in microalgae as the C3 compound phosphoglyceric acid1. The enzyme responsible for C3 carbon fixation, ribulose-1,5-bisphosphate carboxylase (Rubisco), however, requires inorganic carbon in the form of CO2 (ref. 2), and Rubisco enzymes from diatoms have half-saturation constants for CO2 of 30–60 µM (ref. 3). As a result, diatoms growing in seawater that contains about 10 µM CO2 may be CO2 limited4. Kinetic and growth studies have shown that diatoms can avoid CO2 limitation5,6,7, but the biochemistry of the underlying mechanisms remains unknown. Here we present evidence that C4 photosynthesis supports carbon assimilation in the marine diatom Thalassiosira weissflogii, thus providing a biochemical explanation for CO2-insensitive photosynthesis in marine diatoms. If C4 photosynthesis is common among marine diatoms, it may account for a significant portion of carbon fixation and export in the ocean, and would explain the greater enrichment of 13C in diatoms compared with other classes of phytoplankton. Unicellular C4 carbon assimilation may have predated the appearance of multicellular C4 plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Calvin, M. et al. Carbon dioxide assimilation in plants. Symp. Soc. Exp. Biol. 5, 284–305 ( 1951).
Cooper, T. G., Filmer, D., Wishnick, M. & Lane, M. D. The active species of “CO2” utilized by ribulose diphosphate carboxylase. J. Biol. Chem. 244, 1081– 1083 (1969).
Badger, M. R. et al. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 76, 1052–1071 (1998).
Riebesell, U., Wolf-Gladrow, D. A. & Smetacek, V. S. Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361, 249– 251 (1993).
Colman, B. & Rotatore, C. Photosynthetic inorganic carbon uptake and accumulation in two marine diatoms. Plant Cell Envir. 18, 919–924 ( 1995).
Korb, R. E., Saville, P. J., Johnston, A. M. & Raven, J. A. Sources of inorganic carbon for photosynthesis by three species of marine diatom. J. Phycol. 33, 433– 440 (1997).
Tortell, P. D., Reinfelder, J. R. & Morel, F. M. M. Active uptake of bicarbonate by diatoms. Nature 390, 243–244 ( 1997).
Morris, I. in Primary Productivity in the Sea (ed. Falkowski, P. G.) 139– 159 (Plenum, New York, 1980).
Mortain-Bertrand, A., Descolas-Gros, C. & Jupin, H. Short-term 14C incorporation in Skeletonema costatum (Greville) Cleve (Bacillariophyceae) as a function of light regime. Phycologia 26, 262–269 (1987).
Falkowski, P. G. & Raven, J. A. Aquatic Photosynthesis (Blackwell, Malden, 1997).
Cooper, T. G. & Wood, H. G. The carboxylation of phosphoenolpyruvate and pyruvate. J. Biol. Chem. 246, 5488– 5490 (1971).
Ausenhus, S. L. & O'Leary, M. H. Hydrolysis of phosphoenolpyruvate catalysed by phophoenolpyruvate carboxylase from Zea mays. Biochem. 31, 6427– 6431 (1992).
Hatch, M. D. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim. Biophys. Acta 895, 81–106 (1987).
Morel, F. M. M. et al. Zinc and carbon co-limitation of marine phytoplankton. Nature 369, 740–742 ( 1994).
Lane, T. W. & Morel, F. M. M. Regulation of carbonic anhydrase expression by Zn, Co, and CO2 in the marine diatom Thalassiosira weissflogii. Plant Physiol. 123, 345 –352 (2000).
Hatch, M. D. & Burnell, J. N. Carbonic anhydrase activity in leaves and its role in the first step of C4 photosynthesis. Plant Physiol. 93, 825–828 (1990).
Slack, C. R., Hatch, M. D. & Goodchild, D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem. J. 114, 489–498 (1969).
Magnin, N. C., Cooley, B. A., Reiskind, J. B. & Bowes, G. Regulation and localization of key enzymes during the induction of Kranz-less, C4-type photosynthesis in Hydrilla verticillata. Plant Physiol. 115, 1681–1689 (1997).
Boyd, P. & Newton, P. Evidence of the potential influence of planktonic community structure on the interannual variability of particulate organic carbon flux. Deep-Sea Res. 42, 619 –639 (1995).
Fry, B. & Wainright, S. C. Diatom sources of 13C-rich carbon in marine food webs. Mar. Ecol. Prog. Ser. 76, 149–157 ( 1991).
Hayes, J. M., Strauss, H. & Kaufman, A. J. The abundance of C-13 in marine organic matter and isotopic fractionation in the global biogeochemical cycle of carbon during the past 800 Ma. Chem. Geol. 161, 103– 125 (1999).
Medlin, L. K., Kooistra, W. H. C. F., Gersonde, R., Sims, P. A. & Wellbrock, U. Is the origin of the diatoms related to the end-permian mass extinction? Nova Hedwigia. 65, 1–11 (1997).
Berner, R. A. Atmospheric carbon dioxide levels over phanerozoic time. Science 249, 1382–1386 ( 1990).
Ehleringer, J. R. & Monson, R. K. Evolutionary and ecological aspects of photosynthetic pathway variation. Annu. Rev. Ecol. Syst. 24, 411–439 (1993).
Price, N. M. et al. Preparation and chemistry of the artificial algal culture medium Aquil. Biol. Oceanogr. 6, 443– 461 (1988/89).
Wilbur, K. M. & Anderson, N. G. Electrometric and colourimetric determination of carbonic anhydrase. J. Biol. Chem. 176, 147–154 (1948).
Streamer, M., McNell, Y. R. & Yellowlees, D. Photosynthetic carbon dioxide fixation in zooxanthellae. Mar. Biol. 115, 195–198 (1993).
O'Leary, M. H., Rife, J. E. & Slater, J. D. Kinetic and isotopic effect studies of maize phosphenolpyruvate carboxylase. Biochem. 20, 7308– 7314 (1981).
Acknowledgements
We wish to thank M. Spring for help with the enzyme analyses; S.I. Chang, C.-W. Fan; and P.D. Tortell for help with the pulse-chase experiments; I. Schaperdoth for help with the cell fractionation; and T. Lane and K. Keller for useful comments. Funding from the Center for Environmental Bioinorganic Chemistry supported by NSF and DOE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reinfelder, J., Kraepiel, A. & Morel, F. Unicellular C4 photosynthesis in a marine diatom. Nature 407, 996–999 (2000). https://doi.org/10.1038/35039612
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35039612
This article is cited by
-
Unveiling the underlying molecular mechanisms of high lutein production efficiency in Chlorella sorokiniana FZU60 under a mixotrophy/photoautotrophy two-stage strategy by transcriptomic, physiological, and biochemical analyses
Biotechnology for Biofuels and Bioproducts (2023)
-
Temperature is a better predictor of stable carbon isotopic compositions in marine particulates than dissolved CO2 concentration
Communications Earth & Environment (2022)
-
Nano-ecotoxicology in a changing ocean
SN Applied Sciences (2022)
-
Proteomic and biochemical responses to different concentrations of CO2 suggest the existence of multiple carbon metabolism strategies in Phaeodactylum tricornutum
Biotechnology for Biofuels (2021)
-
Exopolysaccharides directed embellishment of diatoms triggered on plastics and other marine litter
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.