Abstract
Most lichenized fungi produce abundant sexual structures, and in many species sexual spores seem to provide the only means of dispersal. For example, 90% of lichens found in Great Britain and Ireland2 produce ascomata (fruit bodies) containing sexually derived ascospores, whereas only 29% form symbiotic vegetative propagules. Sex in lichenized fungi has been assumed to equate with outcrossing3, but failure to induce sexuality in vitro has prevented experimental investigation of their breeding systems.
Similar content being viewed by others
Main
We avoided this problem by using molecular markers to elucidate the sexual cycle. We compared the DNA fingerprints of single-spore progeny from a single ascoma: the presence or absence of genetic variation between sibling spores would show whether outcrossing (heterothallism) or self-fertilization (homothallism: allowing the fusion of two identical nuclei during meiosis) had occurred.
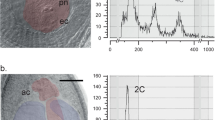
The crustose lichens Graphis scripta (order: Ostropales) and Ochrolechia parella (order: Pertusariales) fruit abundantly, but neither produces symbiotic vegetative propagules (Fig. 1a,b). We collected discrete, symmetrical lichen thalli at locations more than 10 m apart in south Wales, and induced excised individual ascomata to discharge spores. Extraction of DNA from cultured mycelia derived from single spores gave 218–263 randomly amplified polymorphic DNA (RAPD) markers4 for each isolate. In both species, these markers revealed that spores from the same ascoma are genetically uniform (Fig. 1c), providing compelling evidence of homothallism.
a, b, The crustose lichens Graphis scripta and Ochrolechia parella growing on tree bark and maritime rocks, respectively (scale bars, 10 mm). Both species develop abundant ascomata (elongate in G. scripta and disc-shaped in O. parella), in which sexual ascospores are produced. c, Randomly amplified polymorphic DNA (RAPD) markers generated with primer OPAJ-03 showing uniformity in a sample of ten single-spore isolates from one ascoma of G. scripta. A set of ten progeny was collected from each of three separate thalli of both species and assessed for variability in RAPD profiles using a minimum of 30 primers (Operon Technologies). Consistent uniformity among the RAPD markers indicated that for both species all spores from the same ascoma were genetically identical. d, RAPD markers generated with primer OPAX-12 showing polymorphisms between isolates from four different thalli (I–IV) of G. scripta but uniformity among spores from 2–4 different ascomata (replicates A–D) on the same thallus. Eight thalli of G. scripta were compared, five of which were collected from the same woodland. For O. parella, 3–4 ascomata on each of three thalli were compared and polymorphisms found between both thalli and ascomata on the same thallus. M, size markers in kilobases; C, water control.
This breeding system probably serves an ecological function similar to that of self-pollination in flowering plants5,6,7. It may confer a selective advantage by facilitating high spore output without the need for outcrossing; it would also promote the development of a lichen population from a single pioneer spore after dispersal into a new site. Genetic stability may be advantageous in abiotically extreme but relatively undisturbed habitats in which there is a low intensity of biotic interactions because it perpetuates successful genotypes adapted to the prevailing environmental regimes. Homothallism also retains certain benefits of sexual over asexual reproduction, including opportunistic outcrossing, as obligate selfing is rare in homothallic fungi8.
Although we found both lichen fungi to be homothallic, the ascospore offspring derived from different conspecific thalli were genetically distinct (Fig. 1d). The average RAPD genetic divergence9 observed in five isolates of G. scripta from one woodland was 15.2% (according to a neighbour-joining analysis of Jaccard's coefficient of band matching). Furthermore, ascospore progeny from different ascomata on ‘single’ thalli of O. parella showed RAPD polymorphisms, indicating that these lichen thalli may have been composed of at least two sexually active fungal genotypes.
Comparable variation within individual thalli was not detected in G. scripta (Fig. 1d). This difference is probably related to habitat: G. scripta is a pioneer, with thalli that probably arise from single spores, whereas the higher rates of spore deposition in the more densely occupied habitats of O. parella may cause frequent mergers between developing thalli. These ‘mechanical hybridizations’ are probably very common10.
If homothallism is a general characteristic of lichen-forming fungi, including those in the order Lecanorales (which contains the most lichen-forming fungal species), it may explain the ecological paradox of the persistence of fruiting lichens in severe habitats where a stable, highly adapted genetic line would be most advantageous11. It has been shown that sexual reproduction is predominant in lichen communities growing at the frontiers of terrestrial life in polar regions12,13. Lichens may thus provide a model for the evolution of breeding systems in extreme environments.
References
Kershaw, K. A. Physiological Ecology of Lichens (Cambridge Univ. Press, Cambridge, 1985).
Purvis, O. W. et al. (eds) The Lichen Flora of Great Britain and Ireland (Natural History Museum Publications, London, 1992).
Hestmark, G. Oecologia 92, 305–312 ( 1992).
Murtagh. G. J., Dyer, P. S., McClure, P. C. & Crittenden, P. D. Lichenologist 31, 257–267 (1999).
Jarne, P. & Charlesworth, D. Annu. Rev. Ecol. Syst. 24, 441–466 (1993).
Jaine, S. K. Annu. Rev. Ecol. Syst. 7, 469–496 (1976).
Richards, A. J. Plant Breeding Systems 2nd edn (Chapman & Hall, London, 1997).
Burnett, J. H. Mycogenetics: An Introduction to the General Genetics of Fungi (Wiley, London, 1975).
Weising, K., Nybom, H., Wolff, K. & Meyer, W. DNA Fingerprinting in Plants and Fungi (CRC, Boca Raton, 1995).
Jahns, H. M. & Ott, S. Bibl. Lichenol. 67, 49–67 (1997).
Maynard Smith, J. The Evolution of Sex (Cambridge Univ. Press, Cambridge, 1978).
Fahselt, D., Maycock, P. F. & Wong, P. Y. Lichenologist 21, 343– 353 (1989).
Sancho, L. G. & Valladares, F. Polar Biol. 13, 227–233 ( 1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murtagh, G., Dyer, P. & Crittenden, P. Sex and the single lichen. Nature 404, 564 (2000). https://doi.org/10.1038/35007142
Issue Date:
DOI: https://doi.org/10.1038/35007142
This article is cited by
-
Species in lichen-forming fungi: balancing between conceptual and practical considerations, and between phenotype and phylogenomics
Fungal Diversity (2021)
-
The lichen symbiosis re-viewed through the genomes of Cladonia grayi and its algal partner Asterochloris glomerata
BMC Genomics (2019)
-
Twenty-seven modes of reproduction in the obligate lichen symbiosis
Brittonia (2018)
-
Panmixia and dispersal from the Mediterranean Basin to Macaronesian Islands of a macrolichen species
Scientific Reports (2017)
-
Microsatellite analyses of the Antarctic endemic lichen Buellia frigida Darb. (Physciaceae) suggest limited dispersal and the presence of glacial refugia in the Ross Sea region
Polar Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.