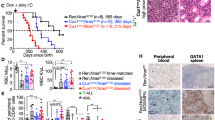
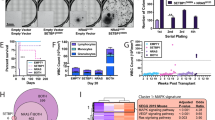
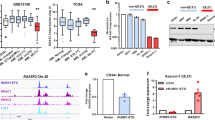
Abstract
PML and Tif1a are fused to RARA and Braf, respectively, resulting in the production of PML-RARα and Tif1α-B-Raf (T18) oncoproteins. Here we show that PML, Tif1α and RXRα/RARα function together in a transcription complex that is dependent on retinoic acid (RA). We found that PML acts as a ligand-dependent coactivator of RXRα/RARα. PML interacts with Tif1α and CBP. In Pml–/– cells, the RA-dependent induction of genes such as RARB2 and the ability of Tif1α and CBP to act as transcriptional coactivators on RA are impaired. We show that both PML and Tif1α are growth suppressors required for the growth-inhibitory activity of RA. T18, similar to PML-RARα, disrupts the RA-dependent activity of this complex in a dominant-negative manner resulting in a growth advantage. Our data define a new pathway for the control of cell growth and tumorigenesis, and provide a new model for the pathogenesis of acute promyelocytic leukaemia (APL).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Smith, M.A., Parkinson, D.R., Cheson, B.D. & Friedman, M.A. Retinoids in cancer therapy. J. Clin. Oncol. 10, 839–864 (1992).
Gudas, L.J., Sporn, M.B. & Roberts, A.B. The Retinoids: Cellular Biology and Biochemistry of the Retinoids 443–520 (Raven Press, New York, 1994).
Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 955–960 (1996).
He, L.-Z., Merghoub, T. & Pandolfi, P.P. In vivo analysis of the molecular pathogenesis of acute promyelocytic leukemia in the mouse and its therapeutic implications. Oncogene 18, 5278–5292 (1999).
Borden, K.L.B. et al. The solution structure of the RING finger domain from the acute promyelocytic leukaemia proto-oncoprotein PML. EMBO J. 14, 1532–1541 (1995).
Perez, A. et al. PML/RAR homodimers: distinct DNA binding properties and heteromeric interactions with RAR. EMBO J. 12, 3171–3182 (1993).
Fagioli, M. et al. Identification of various PML gene isoforms and characterization of their origin and expression pattern. Oncogene 7, 1083–1091 (1992).
Hodges, M., Tissot, C., Howe, K., Grimwade, D. & Freemont, P.S. Structure, organization, and dynamics of promyelocytic leukemia protein nuclear bodies. Am. J. Hum. Genet. 63, 297–304 (1998).
Gaboli, M., Gandini, D., Delva, L., Wang, Z.G. & Pandolfi, P.P. Acute promyelocytic leukemia as a model for cross-talk between interferon and retinoic acid pathways: from molecular biology to clinical applications. Leuk. Lymphoma 30, 11–22 (1998).
Mu, Z.M., Chin, K.V., Liu, J.H., Lozano, G. & Chang, K.S. PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol. Cell. Biol. 14, 6858–6867 (1994).
Wang, Z.G. et al. Role of PML in cell growth and the retinoic acid pathway. Science 279, 1547–1551 (1998).
Wang, Z.-G. et al. Pml is essential for multiple apoptotic pathways. Nature Genet. 20, 266–271 (1998).
Miki, T. et al. Development of a highly efficient expression cDNA cloning system: application to oncogene isolation. Proc. Natl Acad. Sci. USA 88, 5167–5171 (1991).
Le Douarin, B. et al. The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 14, 2020–2033 (1995).
Klugbauer, S. & Rabes, H.M. The transcription coactivator HTIF1 and a related protein are fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas. Oncogene 18, 4388–4393 (1999).
vom Baur, E. et al. Differential ligand dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 15, 110–124 (1995).
de Thé, H., Vivanco-Ruiz, M.d.M., Tiollais, P., Stunnenberg, H. & Dejean, A. Identification of a retinoic acid responsive element in the retinoic acid receptor β gene. Nature 343, 177–180 (1990).
Rachez, C. et al. A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev. 12, 1787–1800 (1998).
Rachez, C. et al. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 398, 824–828 (1999).
Näär, A.M. et al. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature 398, 828–832 (1999).
Fondell, J.D., Ge, H. & Roeder, R.G. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc. Natl Acad. Sci. USA 93, 8329–8333 (1996).
Chakravati, D. et al. Role of CBP/P300 in nuclear receptor signalling. Nature 383, 99–103 (1996).
Bannister, A.J. & Kouzarides, T. The CBP co-activator is a histone acetyltransferase. Nature 384, 641–643 (1996).
LaMorte, V.J., Dyck, J.A., Ochs, R.L. & Evans, R.M. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc. Natl Acad. Sci. USA 95, 4991–4996 (1998).
Fagioli, M. et al. Cooperation between the RING + B1-B2 and coiled-coil domains of PML is necessary for its effects on cell survival. Oncogene 16, 2905–2913 (1998).
Vallian, S. et al. Modulation of Fos-mediated AP-1 transcription by the promyelocytic leukemia protein. Oncogene 16, 2843–2853 (1998).
Vallian, S., Chin, K.V. & Chang, K.S. The promyelocytic leukemia protein interacts with Sp1 and inhibits its transactivation of the epidermal growth factor receptor promoter. Mol. Cell. Biol. 18, 7147–7156 (1998).
Alcalay, M. et al. The promyelocytic leukemia gene product (PML) forms stable complexes with the retinoblastoma protein. Mol. Cell. Biol. 18, 1084–1093 (1998).
Guiochon-Mantel, A. et al. Effect of PML and PML-RAR on the transactivation properties and subcellular distribution of steroid hormone receptors. Mol. Endocrinol. 9, 1791–1803 (1995).
Liu, M., Iavarone, A. & Freedman, L.P. Retinoid induction of U937 cell differentiation: transcriptional activation of the human p21WAF1/CIP1 gene by retinoic acid receptor. J. Biol. Chem. 271, 31723–31728 (1996).
Pomponi, F. et al. Retinoids irreversibly inhibit in vitro growth of Epstein-Barr virus-immortalized B lymphocytes. Blood 88, 3147–3159 (1996).
Schüle, R. et al. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc. Natl Acad. Sci. USA 88, 6092–6096 (1991).
Kamei, Y. et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85, 403–414 (1996).
Huang, C. et al. Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc. Natl Acad. Sci. USA 94, 5826–5830 (1997).
Nason-Burchenal, K. et al. Interferon augments PML and PML/RARα expression in normal meyloid and acute promyelocytic cells and cooperates with all-trans retinoic acid to induce maturation of a retinoid resistant promyelocytic cell line. Blood 88, 3926–3936 (1996).
Flenghi, L. et al. Characterization of a new monoclonal antibody (PG-M3) directed against the aminoterminal portion of the PML gene product: immunocytochemical evidence for high expression of PML proteins on activated macrophages, endothelial cells, and epithelia. Blood 85, 1871–1880 (1995).
de Thé, H., Marchio, A., Tiollais, P. & Dejean, A. Differential expression and ligand regulation of the retinoic acid receptor α and β genes. EMBO J. 8, 429–433 (1989).
Vasios, G.W., Gold, J.D., Petkovitch, M., Chambon, P. & Gudas, L.J. A retinoic acid-responsive element is present in the 5′ flanking region of the laminin B1 gene. Proc. Natl Acad. Sci. USA 86, 9099–9103 (1989).
Manshouri, T. et al. Downregulation of RARα in mice by antisense transgene leads to a compensatory increase in RARβ and RARα and development of lymphoma. Blood 89, 2507–2515 (1997).
David, G., Terris, B., Marchio, A., Lavau, C. & Dejean, A. The acute promyelocytic leukemia PML-RARα protein induces hepatic preneoplastic and neoplastic lesions in transgenic mice. Oncogene 14, 1547–1554 (1997).
He, L.Z. et al. Distinct interactions of PML-RARα and PLZF-RARα with co-repressors determine differential responses to RA in APL. Nature Genet. 18, 126–135 (1998).
Grignani, F. et al. Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukaemia. Nature 391, 815–818 (1998).
Lin, R.J. et al. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 391, 811–814 (1998).
Boddy, M.N., Howe, K., Etkin, L.D., Solomon, E. & Freemont, P.S. PIC 1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene 13, 971–982 (1996).
Filvaroff, E., Stern, D.F. & Dotto, G.P. Tyrosine phosphorylation is an early and specific event involved in primary keratinocyte differentiation. Mol. Cell. Biol. 10, 1164–1173 (1990).
Glass, C.K., Holloway, J.M., Devary, O.V. & Rosenfeld, M.G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell 54, 313–323 (1988).
Jin, S. & Scotto, K.W. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol. Cell. Biol. 18, 4377–4384 (1998).
Qin, X.Q., Chittenden, T., Livingston, D. & Kaelin, W.G. Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 6, 953–964 (1992).
Acknowledgements
We thank M. Barna, C. Chomienne, T. Delohery, G.P. Dotto, M. Fagioli, P.S. Freemont, F. Grosveld, L. Longo, L. Luzzatto, T. Miki, P.G. Pelicci, V. Richon, P. Sassone-Corsi, K. Scotto, A. Simeone and E. Singh for help, materials and advice. Partially supported by the "Ligue Nationale contre le Cancer" of France (L.D.) and the American-Italian Cancer Foundation (D.G.). P.P.P. is a scholar of the Leukemia Society of America. Supported by the NCI (CA-08748) and NIH (CA71692 and CA74031 to P.P.P.) and the Human Frontiers Science Program (to C.R. and L.P.F.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, S., Delva, L., Rachez, C. et al. A RA-dependent, tumour-growth suppressive transcription complex is the target of the PML-RARα and T18 oncoproteins. Nat Genet 23, 287–295 (1999). https://doi.org/10.1038/15463
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/15463
This article is cited by
-
TRIM24 is upregulated in human gastric cancer and promotes gastric cancer cell growth and chemoresistance
Virchows Archiv (2015)
-
Heterogeneity of molecular markers in chronic myelomonocytic leukemia: a disease associated with several gene alterations
Cellular and Molecular Life Sciences (2012)
-
TRIM proteins and cancer
Nature Reviews Cancer (2011)
-
TRIM24 links a non-canonical histone signature to breast cancer
Nature (2010)
-
New insights into the role of PML in tumour suppression
Cell Research (2008)