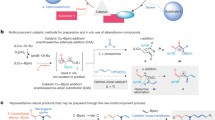
Abstract
Heterogeneous bifunctional catalysts consisting of palladate–osmate and osmate–tungstate developed by the ion exchange on quaternary ammonium salts covalently bound to resin, and their homogeneous bimetallic analogs are evaluated for the synthesis of chiral vicinal diols. The heterogeneous bifunctional catalyst (resin-PdOs) and its homogeneous analog are used in the tandem Heck-asymmetric dihydroxylation (AD) to afford diols with excellent yields and enantiomeric excesses (ee's) in the presence of various co-oxidants. The other bifunctional catalyst (resin-OsW) and its homogeneous analog are used in a simultaneous asymmetric dihydroxylation-N-oxidation of a wide range of olefins to obtain chiral vicinal diols with higher yields and ee's using H2O2 as terminal oxidant. The bifunctional resin catalysts are recovered quantitatively by a simple filtration and reused for a number of cycles with consistent activity.
Similar content being viewed by others
References
A. Domling and I. Ugi, Angew. Chem. Int. Ed. Engl. 32 (1993) 563. (b)C.A. Roessner and A.I. Scott, Chem. Biol. 3 (1996) 325. (c)P.A. Wender, S.T. Handy and D.L. Wright, Chem. Ind 19 (1997) 766.
J.S. Kingsbury, S.B. Garber, J.M. Giftos, B.L. Gray, M.M. Okamoto, R.A. Farrer, J.T. Fourkas and A.H. Hoveda, Angew. Chem. Int. Ed. 40 (2001) 4251. (b)J. Louie, C.W. Bielawski and R.H. Grubbs, J. Am. Chem. Soc. 123 (2001) 11312.
S. Yamasaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc. 123 (2001) 1256.
For bifunctional catalysts see: (a) H.-B. Yu, Q.-S. Hu and L. Pu, J. Am. Chem. Soc. 122 (2000) 6500. (b)M. Shibasaki, Chemtracts-Org. Chem. 12 (1999) 979.(c)G.J. Rowlands, Tetrahedron 57 (2001) 1865. (d)F. Stefan, W. Harald, M.H. Ahmed, E.T. Andrew, R.W. Daniel and L. Thomas, Org. Lett. 4 (2002) 1603.(e)H. Gröger, Chem. Eur. J. 7 (2001) 5247.(f)Y. Hamashima, D. Sawada, H. Nogami, M. Kanai and M. Shibasaki, Tetrahedron 57 (2001) 805.
V.K. Aggarwal, D.M. Dean, A. Meruu and R. Williams, J. Org. Chem. 67 (2002) 510.
H.C. Kolb, M.S. VanNieuwenhze and K.B. Sharpless, Chem. Rev. 94 (1994) 2483. (b)I.E. Marko and J.S. Svendsen in: Comprehensive Asymmetric Catalysis II, eds. E.N. Jacobsen, A. Pfaltz and H. Yamamoto(Springer, Berlin, 1999) p. 723.(c)C. Döbler, G. Mehltretter and M. Beller, Angew. Chem. 111 (1999) 3211.(d)J.S.M. Wai, I.E. Marko, J.S. Svendsen, M.G. Finn, E.N. Jacobsen and K.B. Sharpless, J. Am. Chem. Soc. 111 (1989) 1123.(e)K. Bergstad, S.Y. Jonsson and J.-E. Bäckvall, J. Am. Chem. Soc. 121 (1999) 10424.(f)S.Y. Jonsson, K. Farnegardh and J.-E. Bäckvall, J. Am. Chem. Soc. 123 (2001) 1365.(g)S.Y. Jonsson, H. Adolfsson and J.-E. Bäckvall, Org. Lett. 3 (2001) 3465.(h)A.H. Ell, S.Y. Jonsson, A. Borje, H. Adolfson and J.-E. Bäckvall, Tetrahedron Lett. 42 (2001) 2569.(i)C. Bolm and A. Gerlach, Eur. J. Org. Chem. 1 (1998) 21.(j)P. Salvadori, D. Pini and A. Petri, Synlett 8 (1999) 1181.(k)D.J. Gravert and K.D. Janda, Chem. Rev. 97 (1997) 489.(l)S. Kobayashi, M. Endo and S. Nagayama, J. Am. Chem. Soc. 121 (1999) 11229.
V. VanRheenen, R.C. Kelly and D.F. Cha, Tetrahedron Lett. 17 (1976) 1973. (b)J.S.M. Wai, I.E. Marko, J.S. Svendsen, M.G. Finn, E.N. Jacobsen and K.B. Sharpless, J. Am. Chem. Soc. 111 (1989) 1123. (c)H.C. Kolb and K.B. Sharpless, J. Org. Chem. 59 (1994) 5104. (d)Z.-M. Wang and K.B. Sharpless, J. Org. Chem. 59 (1994) 8302. (e)L. Ahrgren and L. Sutin, Org. Process Res. Dev. 1 (1997) 425. (f)X. Lu, Z. Xu and G. Yang, Org. Process Res. Dev. 4 (2000) 575.
M.P. Singh, H.S. Singh, A.K. Arya, A.K. Singh and A.K. Sisodia, Indian J. Chem. 13 (1975) 112.(b)M. Minata, K. Yamamoto and J. Tsuji, J. Org. Chem. 55 (1990) 766.(c)K.B. Sharpless, W. Amberg, M. Beller, H. Chen, J. Hartung, Y. Kawanami, D. Lubben, E. Manoury, Y. Ogino, T. Shibata and T. Ukita, J. Org. Chem. 56 (1991) 4585.(d)H. Becker and K.B. Sharpless, Angew. Chem. Int. Ed. 35 (1996) 448.
A. Krief, C. Colaux-Castillo, Tetrahedron Lett. 40 (1999) 4189. (b)C. Döbler, G. Mehltretter and M. Seller, Angew. Chem. Int. Ed. 38 (1999) 3026.(c)C. Döbler, G, Mehltretter, U. Sundermeier and M. Beller, J. Am. Chem. Soc. 122 (2000) 10289.(d)C. Döbler, G. Mehltretter, U. Sundermeier and M. Beller, J. Organomet. Chem. 621 (2001) 70.
S. Nagayama, M. Endo and S. Kobayashi, J. Org. Chem. 63 (1998) 6094. (b)S. Kobayashi, M. Endo and S. Nagayama, J. Am. Chem. Soc. 121 (1999) 11229.(c)S. Kobayashi, T. Ishida and R. Akiyama, Org. Lett. 3 (2001) 2649.(d)A. Ryo and S. Kobayashi, Angew. Chem. Int. Ed. Engl. 40(2001)3469.
B.M. Choudary, N.S. Chowdari, S. Madhi and M.L. Kantam, Angew. Chem. Int. Ed. Engl. 40 (2001) 4620. (b)B.M. Choudary, N.S. Chowdari, K. Jyothi and M.L. Kantam, J. Am. Chem. Soc. 124 (2002) 5341.(c)B.M. Choudary, N.S. Chowdari, M.L. Kantam and K.V. Raghavan, J. Am. Chem. Soc. 123 (2001) 9220.
A. Severeyns, D.E. De Vos, L. Fiermans, F. Verpoort, P.J. Grobet and P.A. Jacobs, Angew. Chem. Int. Ed. 40 (2001) 586.
We have also tested Ti(IV) as an ETM, the results are inferior; see: B.M. Choudary, N.S. Chowdary, K. Jyothi, S. Madhi andM.L. KantamAdv. Synth. Catal. 344 (2002) 503.
14.(a)B. Hinzen and S.V. Ley, J. Chem. Soc., Perkin Trans. 1 (1997) 1907. (b)B. Hinzen, R. Lenz and S.V. Ley, Synthesis 7 (1998) 977. (c)S.B. Roscoe, J.M. Frechet, J.F. Walzer and A.J. Dias, Science 280 (1998) 270.
ESCA Pd: G. Kumar, R.J. Blackburn, R.G. Albridge, W.E. Moddeman and M.M. Jones, Inorg. Synth. 11 (1972) 296. (b)K. Kili, L. Hilaire and F.L. Normand, Phys. Chem. Chem. Phys. 1 (1999) 1623. (c)F.B. Verduraz, A. Omar, J. Escard and B. Pontvianne, J. Catal. 53 (1978) 126.
D.L. White S.B. Andrews J.W. Faller R.J. Barrnett (1976) Biochim. Biophys. Acta 436 577
ESCA of W: J.R. Sohn and M.Y. Park, Langmuir 14 (1998) 6140. (b)L. Dong, Y. Hu, F. Xu, D. Lu, B. Xu, Z. Hu and Y. Chen, J. Phys. Chem. B 104 (2000) 78.(c)Y. Peng, Z. Meng, C. Zhong, J. Lu, Z. Yang and Y. Qian, Chem. Lett. (2001) 64.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Choudary, B.M., Madhi, S., Chowdari, N.S. et al. Heterogeneous Bifunctional Catalysts for the Synthesis of Chiral Vicinal Diols. Topics in Catalysis 29, 183–187 (2004). https://doi.org/10.1023/B:TOCA.0000029801.51873.61
Issue Date:
DOI: https://doi.org/10.1023/B:TOCA.0000029801.51873.61