Abstract
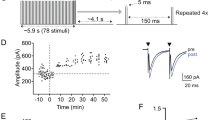
Long-term potentiation in the thalamo-cortical input to the somatosensory cortex barrel field has been reported to be inducible in vitro only during a narrow critical period of the first postnatal week. Here we explored whether this is due to inability of adult synapses to express LTP or lack of appropriate conditions for LTP induction in slice preparations. We recorded thalamo-cortical field potentials (FPs) from the barrel field of chronically prepared adult rats. In the first series, several parameters of conditioning tetanization of thalamus (T) have been tried. Statistically significant LTP of 135–150% relative to the baseline was observed only in rare cases (3/18) so that the mean changes were not statistically significant. In the second series, five trains of 100 Hz stimulation of T were paired with a “reinforcing” stimulation of the lateral hypothalamus (LH). In most cases (9/13), thalamo-cortical FPs were potentiated. The mean post-tetanic amplitude was 238 ± 42% (±SEM relative to the baseline (n= 13). The potentiation persisted for >1 h and typically even further increased when tested 24–48 h later. LTP magnitude strongly correlated with the initial paired-pulse ratio (PPR, coefficient of correlation r= 0.98) so that the LTP magnitude was larger (333 ± 107, n= 6) in cases with PPR > 1.3. The mean PPR tended to decrease after LTP (from 2.05 to 1.65). Altogether the results suggest that LTP is inducible in the thalamo-cortical input to the barrel field of normal adult rats. The dependence of the LTP magnitude upon the initial PPR suggests that inputs with low initial release probability undergo larger LTP. Together with the tendency to a decrease in the PPR this suggests an involvement of presynaptic mechanisms in the maintenance of neocortical LTP.
Similar content being viewed by others
REFERENCES
J. M. Armstrong, M. E. Diamond, and F. F. Ebner, “An innocuous bias in whisker use in adult rats modifies receptive fields of barrel cortex neurons,” J. Neurosci., No. 14, 6978–6991 (1994).
A. Artola and W. Singer, “Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation,” Trends Neurosci., 16, No. 11, 480–487 (1993).
C. H. Bailey, M. Giustetto, Y.-Y. Huang, et al., “Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory?” Nature Rev., No. 1, 11–20 (2001).
I. Bayazitov and A. Kleschevnikov, “Afferent high strength tetanizations favour potentiation of the NMDA vs. AMPA receptor-mediated component of field EPSP in CA1 hippocampal slices of rats,” Brain Res., 866, 188–196 (2000).
M. F. Bear, “NMDA-receptor-dependent synaptic plasticity in the visual cortex,” Prog. Brain Res., 108, 205–218 (1996).
T. V. P. Bliss and G. L. Collingridge, “A synaptic model for memory: long-term potentiation in the hippocampus,” Nature, 361, 31–39 (1993).
T. E. Boyd, C. Trepel, and R. J. Racine, “Cholinergic modulation of neocortical long-term potentiation in the awake, freely moving rat,” Brain Res., 881, No. 1, 28–36 (2000).
P. M. Cahusac, “Synaptic plasticity induced in single neurones of the primary somatosensory cortex in vivo,” Exp. Brain Res., 107, 241–253 (1995).
C. A. Chapman, C. Trepel, T. L. Ivanco, et al., “Changes in field potentials and membrane currents in rat sensorimotor cortex following repeated tetanization of the corpus callosum in vivo,” Cereb. Cortex, 8, No. 8, 730–742 (1998).
S. Choi, J. Klingauf, and R. W. Tsien, “Postfusional regulation of cleft glutamate concentration during LTP at “silent synapses”,” Nat. Neurosci., 3, 330–336 (2000).
M. C. Crair and R. C. Malenka, “A critical period for longterm potentiation at thalamocortical synapses,” Nature, 375, 325–328 (1995).
F. A. Edwards, “Anatomy and electrophysiology of fast central synapses lead to a structural model for long-term potentiation,” Physiol Rev., 75, No. 4, 759–787 (1995).
V. Ego-Stengel, De. Shulz, S. Haidarliu, et al., “Acetylcholinedependent induction and expression of functional plasticity in the barrel cortex of the adult rat,” J. Neurophysiol., 86, No. 1, 422–437 (2001).
V. L. Ezrokhi, V. A. Zosimovskii, V. A. Korshunov, and V. A. Markevich, “Restoration of decaying long-term potentiation in the hippocampal formation by stimulation of neuromodulatory nuclei in freely moving rats,” Neuroscience, 88, No. 3, 741–753 (1999).
D. E. Feldman, R. A. Nicoll, and R. C. Malenka, “Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses,” J. Neurobiol., 41, 92–101 (1999).
K. Fox, “The role of excitatory amino acid transmission in development and plasticity of SI barrel cortex,” Prog. Brain Res., 108, 219–234 (1996).
S. Frey, J. Bergado-Rosado, T. Seidenbecher, et al., “Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP,” J. Neurosci., 21, No. 10, 3697–3703 (2001).
U. Frey and R. G. M. Morris, “Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation,” Trends Neurosci., 21, 181–188 (1998).
D. J. Froc, C. A. Chapman, C. Trepel, and R. J. Racine, “Longterm depression and depotentiation in the sensorimotor cortex of the freely moving rat,” J. Neurosci., 20, 438–445 (2000).
S. Gasparini, C. Saviane, L. L. Voronin, and E. Cherubini, “Silent synapses in the developing hippocampus: Lack of functional AMPA receptors or low probability of glutamate release?” Proc. Nat. Acad. Sci. USA, 97, 9741–9746 (2000).
A. J. Heinen and M. F. Bear, “Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo,” J. Neurosci., 21, 9801–9813 (2001).
J. T. R. Isaac, M. C. Crair, R. A. Nicoll, and R. C. Malenka, “Silent synapses during development of thalamocortical inputs,” Neuron., 18, 269–280 (1997).
A. M. Kleschevnikov, M. Sokolov, U. Kuhnt, et al., “Changes in paired-pulse facilitation correlated with induction of long-term potentiation in area CA1 of rat hippocampal slices,” Neuroscience, 76, 829–843 (1997).
A. M. Kleschevnikov, U. Kuhnt, and L. L. Voronin, “Quantal analysis of a late phase of long-term potentiation in the guinea pig hippocampal slices: sharp microelectrode recordings,” Neurosci. Res. Commun., 30, 7–25 (2002).
J. F. R. Konig and R. A. Klippel, The Rat Brain. A Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem, Acad. Press, Baltimore (1963), 162 pp.
U. Kuhnt and L. L. Voronin, “Interaction between paired-pulse facilitation and long-term potentiation in area CA1 of guinea pig hippocampal slices: application of quantal analysis,” Neuroscience, 62, 391–397 (1994).
D. M. Kullmann, “Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation,” Neuron, 12, 1111–1120 (1994).
A. Larkman, T. Hannay, K. Stratford, and J. Jack, “Presynaptic release probability influences the locus of long-term potentiation,” Nature, 360, 70–73 (1992).
R. C. Malenka and R. A. Nicoll, “Long-term potentiation. — A decade of progress?” Science, 285, 1870–1874 (1999).
R. Malinow, Z. F. Mainen, and Y. Hayashi, “LTP-mechanisms: from silence to four-lane traffic,” Curr. Opin. Neurobiol., 10, 352–357 (2000).
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, F1: Acad. Press, Orlando (1986), 136 pp.
R. J. Racine, G. C. Teskey, D. Wilson, et al., “Post-activation potentiation and depression in the neocortex of the rat: II. Chronoc prepapations,” Brain Res., 637, 83–96 (1994).
R. J. Racine, C. A. Chapman, C. Trepel, et al., “Post-activation potentiation in the neocortex: and depression in the neocortex of the rat: IV. Multiple sessions required for induction of long-term potentiation in the chronic preparation,” Brain Res., 702, 87–93 (1994).
J. J. Renger, C. Egles, and G. Liu, “A developmental switch in neurotransmitter flux enhances synaptic efficacy by affecting AMPA receptor activation,” Neuron, 29, 469–484 (2001).
S. Rumpel, H. Hatt, and K. Gottmann, “Silent synapses in developing rat visual cortex: evidence for postsynaptic expression of synaptic plasticity,” J. Neurosci., 18, 8863–8874 (1998).
P. E. Schulz, E. P. Cook, and D. Johnson, “Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation,” J. Neurosci., 14, 5325–5337 (1994).
M. V. Sokolov, A. V. Rossokhin, T. Behnisch, et al., “Interaction between paired-pulse facilitation and long-term potentiation of minimal EPSPs in rat hippocampal slices: a patch clamp study,” Neuroscience, 85, 1–13 (1998).
N. Torii, T. Tsumoto, L. Uno, et al., “Quantal analysis suggests presynaptic involvement in expression of neocortical short-and long-term depression,” Neuroscience, 79, 317–321 (1997).
C. Trepel and R. J. Racine, “Long-term potentiation in the neocortex of the adult, freely moving rat,” Cereb. Cortex, 8, 719–729 (1998).
T. Tsumoto, “Long-term potentiation and long-term depression in the neocortex,” Prog. Neurobiol., 39, 209–228 (1992).
M. Volgushev, L. L. Voronin, M. Chistiakova, and W. Singer, “Relations between long-term synaptic modifications and pairedpulse interactions in the rat neocortex,” Eur. J. Neurosci., 7, 1751–1760 (1997).
L. L. Voronin, “A study of synaptic plasticity at archicortical and neocortical levels,” Neurophysiology, 16, 651–665 (1984).
L. L. Voronin, “On the quantal analysis of hippocampal long-term potentiation and related phenomena of synaptic plasticity,” Neuroscience, 56, 275–304 (1993).
L. L. Voronin and S. V. Ioffe, “Changes in unit postsynaptic responses at sensorimotor cortex with conditioning in rabbits,” Acta Neurobiol Exp, Warsz, 34, 504–513 (1974).
L. L. Voronin, U. Kuhnt, and G. Hess, “A quantum analysis of the long-term post-tetanic changes in the minimal postsynaptic potentials in surviving hippocampal slices,” Neirofiziologiya/Neurophysiology, 22, No. 6, 752–761 (1990).
L. L. Voronin and V. A. Markevich, “Conditioned reflex analogue with registration of pyramidal tract response to direct cortical stimulation,” Dokl. Biol. Sci., 253, 1005–1009 (1980).
L. L. Voronin and V. A. Markevich, “A neural analog of conditioning: modifications of pyramidal tract response,” in: Conditioning: Representation of Involved Neural Functions. Advances in Behavioral Biology, C. Woody (ed.), 26, 663–676, Plenum, N. Y. (1982).
L. Voronin, A. Byzov, A. Kleschevnikov, et al., “Neurophysiological analysis of long-term potentiation in mammalian brain,” Behav. Brain Res., 66, 45–52 (1995).
T. A. Woolsey and H. Van der Loos, “The structural organization of layer IV in the somatosensory region (SI) of the mouse cerebral cortex,” Brain Res., 17, 204–242 (1970).
R. S. Zucker, “Short-term synaptic plasticity,” Ann. Rev. Neurosci., 12, 13–31 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ezrokhi, V.L., Korshunov, V.A., Markevich, V.A. et al. Stimulation of the Lateral Hypothalamus Provokes the Initiation of Robust Long-Term Potentiation of the Thalamo-Cortical Input to the Barrel Field of the Adult, Freely Moving Rat. Neurosci Behav Physiol 34, 919–927 (2004). https://doi.org/10.1023/B:NEAB.0000042651.41720.82
Issue Date:
DOI: https://doi.org/10.1023/B:NEAB.0000042651.41720.82