Abstract
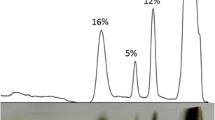
Butyrylcholinesterase (BChE; E.C. 3.1.1.8.) was 260-fold purified from soluble fraction of rat intestine. The enzyme was composed of tetrameric globular form by nonreducing electrophoresis. Optimum pH value was determined as 7.2 after zero buffer extrapolation. Optimum temperature was examined as 37°C after zero time extrapolation. The enzyme showed marked substrate activation with positively charged, acyl-choline substrates. As a measure of catalytic efficiency, k cat/K m values were determined as 16,210, 25,650, and 46,150 for acetylthiocholine (ATCh), propionylthiocholine (PTCh), and butyrylthiocholine (BTCh), respectively. When the catalytic efficiencies are compared, soluble isoform of rat intestinal BChE became increasingly efficient as the size of the acyl portion of the substrate increases; BTCh > PTCh > ATCh. Differently, the enzyme showed substrate inhibition with benzoylcholine (BzCh) and a k cat/K m value of 21,190 was found. Triton X-100 inhibited more efficiently the rat intestinal BChE soluble isoform than it did the human serum BChE.
Similar content being viewed by others
References
Bodur, E., Cokugras, A. N., and Tezcan, E. F. (2001). Arch. Biochem. Biophys. 386: 25–29.
Boeck, A. T., Schopfer, L. M., and Lockridge, O. (2002). Biochem. Pharmacol. 63: 2101–2110.
Bradford, M. M. (1976). Anal. Biochem. 72: 248–254.
Brown, S. S., Kalow, W., Whittaker, M., and Woronick, C. L. (1981). Adv. Clin. Chem. 22: 1–123.
Cokugras, A. N., and Tezcan, E. F. (1993). Int. J. Biochem. 25: 1115–1120.
Cokugras, A. N., and Tezcan, E. F. (1997). Gen. Pharmac. 29: 835–838.
Dave, K. R., Syal, A. R., and Katyare, S. S. (2000). Z. Naturforsch. 55c: 100–108.
Ellman, G. L., Courtney, K. D., Andres, V., and Featherstone, R. M. (1961). Biochem. Pharmacol. 7: 88–95.
Grunwald, J., Marcus, D., Papier, Y., Raveh, L., Pittel, Z., and Ashani, Y. (1997). J. Biochem. Biophys. Methods 34: 123–135.
Hoffman, R. S., Morasco, R., and Goldfrank, L. R. (1996). J. Toxicol-Clin. Toxic. 34: 259–266.
Juul, P. (1968). Clin. Chim. Acta 19: 205–213.
Laemmli, U. K. (1970). Nature 227: 680–685.
Lockridge, O. (1990). Pharmac. Ther. 47: 35–60.
Lockridge, O., Mottershaw-Jackson, N., Eckerson, H. W., and LaDu, B. N. (1996). J. Pharmac. Exp. 215: 1–8.
Masson, P., Froment, M. T., Fortier, P. L., Visicchio, J. E., Bartels, C. F, Lockridge, O. (1998). Biochem. Biophys. Acta 1387: 41–52.
Masson, P., Xie, W., Froment, M-T., and Lockridge, O. (2001). Biochim. Biophys. Acta 1544: 166–176.
Masson, P., Schopfer, L. M., Bartels, C. F., Froment, M-T., Ribes, F., Nachon, F., et al. (2002). Biochim. Biophys. Acta 1594: 313–324.
Massoulie, J., Pezzementi, L., Bon, S., Krejci, E., and Vallette, F. M. (1992). Neurobiol. 41: 31–91.
McPhalen, C. A., Strynadka, C. Y., and James, M. N. G. (1991). Adv. Prot. Chem. 42: 77–144.
Raveh, L., Grunwald, J., Marcus, D., Papier, Y., Cohen, E., and Ashani, Y. (1993). Biochem. 45: 2465–2474.
Raveh, L., Grauer, E., Grunwald, J., Cohen, E., and Ashani, Y. (1997). Toxicol. Appl. Pharmacol. 145: 43–53.
Sarkarati, B., Cokugras, A. N., and Tezcan, E. F. (1999). Comp. Biochem. Biophys. Part C 122: 181–190.
Segel, I. H. (1975). Enzyme Kinetics, Wiley Interscience, Toronto, pp. 246–464.
Sine, J-P., Toutant, J-P, Weigel, P., and Colas, B. (1992). Biochem. 31: 10893–10900.
Sine, J-P. and Colas, B. (1996). Int. J. Biochem. Cell Biol. 28: 581–589.
Sun, H., Yazal, J. E., Lockridge, O., Schopfer, L. M., Brimijoin, S., and Pang, Y. P. (2001). J. Biol. Chem. 276: 9330–9336.
Tune, K. A., Levin, E., and Svesson, L-A. (1988). Biochem. Pharmacol. 37: 3867–3876.
Xie, W., Altamiro, C. V., Bartels, C. F., Speirs, R. J., Cashman, J. R., and Lockridge, O. (1999). Mol. Pharmacol. 55: 83–91.
Rights and permissions
About this article
Cite this article
Yıldız, Ö., Bodur, E., Çokugraş, A.N. et al. Partial Purification and Characterization of Soluble Isoform of Butyrylcholinesterase from Rat Intestine. J Protein Chem 23, 143–151 (2004). https://doi.org/10.1023/B:JOPC.0000020081.36183.b8
Issue Date:
DOI: https://doi.org/10.1023/B:JOPC.0000020081.36183.b8