Abstract
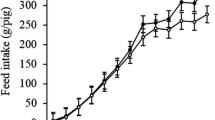
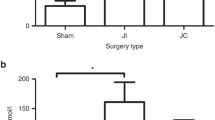
Adaptation of the residual small bowel following resection is dependent on luminal and humoral factors. We aimed to establish if circulating levels of glucagon-like peptide (GLP-2) change under different dietary regimens following resection and to determine if there is a relationship between plasma GLP-2 levels and markers of intestinal adaptation. Four-week-old piglets underwent a 75% proximal small bowel resection (n=31) or transection (n=14). Postoperatively they received either pig chow (n=14), nonpolymeric (elemental) infant formula (n=7), or polymeric infant formula alone (n=8) or supplemented either with fiber (n=6) or with bovine colostrum protein concentrate (CPC; n=10) for 8 weeks until sacrifice. Plasma GLP-2 levels were measured at weeks 0, 2, 4, and 8 postoperatively. In addition, end-stage parameters were studied at week 8 including weight gain, ileal villus height, crypt depth, and disaccharidase levels. Plasma GLP-2 levels were higher in resected animals compared to transected animals fed the same diet. Plasma GLP-2 levels were significantly increased in the colostrum protein isolate-supplemented animals following resection compared to all other diet groups. The increase in plasma GLP-2 (pM) was greatest in the first 2 weeks postresection (week 0, 15.5; week 2, 30.9), followed by a plateau at weeks 2 to 4 and a decrease in GLP-2 levels from week 4 to week 8. At week 8, no relationships were found between the plasma GLP-2 levels and the measurements of weight gain, villus height, lactase, sucrase, maltase, crypt depth, or villus/crypt ratio. Plasma GLP-2 levels increase in the first weeks following massive small intestinal resection. The increase in plasma GLP-2 levels was enhanced by supplementation of the diet with CPC. The changes in GLP-2 levels observed in this study may suggest that GLP-2 plays a role in the adaptive response in the intestine following resection in this preclinical model.
Similar content being viewed by others
REFERENCES
Vanderhoof JA, Langas AN: Short bowel syndrome in children and infants. Gastroenterology 113:1767-1778, 1997
Ziegler MM: Short bowel syndrome: Remedial features that influence outcome and the duration of parenteral nutrition. J Pediatr 131:335-336, 1997
Vanderhoof JA: Short bowel syndrome in children and small bowel transplantation. Pediatr Clin North Am 43:533-550, 1996
Lipman TO: Home artificial nutrition. Curr Opin Clin Nutr Met Care 2:387-393, 1999
Vanderhoof JA: Short bowel syndrome and intestinal adaptation. In Pediatric Gastrointestinal Disease, 3rd ed. WA Walker, PR Durie, JR Hamilton, et al. (eds). Ontario, BC Decker, 2000, pp 583-602
Thompson JS: Can the intestine adapt to a changing environment? Gastroenterology 113:1402-1412, 1997
Wolvekamp MCJ, Heineman E, Taylor RG, Fuller PJ: Towards understanding the process of intestinal adaptation. Dig Dis 14:59-72, 1996
Taylor RG, Fuller PJ: Humoral factors in intestinal adaptation. In The Gut as an Endocrine Organ. Balliere's Clin Endocrinol Metab 8:165-184, 1994
Scott RB, Kirk D, MacNaughton WK, Meddings JB: GLP-2 augments the adaptive response to massive small bowel resection in rat. Am J Physiol 275:G911-G921, 1988
Drucker DJ, DeForest L, Brubaker PL: Intestinal response to growth factors administered alone or in combination with human [Gly2] glucagon-like peptide 2. Am J Physiol 273:G1252-G1262, 1997
Tsai CH, Hill M, Asa SL, Brubaker PL, Drucker DJ: Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol 273:E77-E84, 1997
Brubaker PL, Crivici A, Izzo A, Ehrlich P, Tsai CH, Drucker DJ: Circulating and tissue forms of the intestinal growth factor, glucagonlike peptide-2. Endocrinology 138:4837-4834, 1997
Munroe DG, Cupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboy RK, Chgen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A: Prototypic G proteincoupled receptor for the intestinotropic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96:1569-1577, 1999
Drucker DJ: Glucagon-like peptide 2. TEM 10:153-156, 1999
Xiao Q, Boushey RP, Cino M, Drucker DJ, Brubaker PL: Circulating levels of glucagon-like peptide-2 in human subjects with inflammatory bowel disease. Am J Physiol 278(4):R1057-R1063, 2000
Jeppesen PB, Hartmann B, Hansen BS, Thulesen J, Holst JJ, Mortensen PB: Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut 45(4):559-563, 1999
Dunphy J, Fuller PJ: "Enteroglucagon," bowel growth and GLP-2. Mol Cell Endocrinol 132:7-11, 1997
Baksheev L, Fuller PJ: Hormonal control in intestinal adaptation. Trends Endocrinol Metab 11:401-405, 2000
Benjamin MA, Mc Kay DM, Yang P-C, Cameron H, Perdue MH: Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut 47(1):112-119, 2000
Cheeseman CI, O'Neill D: Bacterial D-glucoase transport activity along the crypt-villus axis in rat jejunum and upregulation induced by gastric inhibitory peptide and glucagon-like peptide-2. Exp Physiol 83(5):605-616, 1998
Dunphy Jl, Justice FA, Taylor RG, Fuller PJ: MRNAlevels of dipeptidyl transferase peptidase IV decrease during intestinal adaptation. J Surg Res 87:130-133, 1999
Xiao Q, Boushey RP, Drucker DJ, Brubaker PL: Secretion of the intestinotrophic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 117(1):99-105, 1999
Hartmann B, Thulesen J, Kissow H, Thulesen S, Orskov C, Ropke C, Poulsen SS, Holst JJ: Dipeptidyl peptidase IV (DPP-IV) inhibition enhances the intestinotrophic effect of glucagon-like peptide-2 in rats and mice. Endocrinology 141:4013-4020, 2000
Alavi K, Yu D, Schwartz MZ: Glucagon-like peptide-2 enhances intestinal function following massive small bowel resection. Gastroenterology 114(4):G4717, 1998
Ljungmann K, Hartmann B, Kissmeyer-Nielsen P, Flyvbjerg A, Holst JJ, Laurberg S: Time-dependent intestinal adaptation and GLP-2 alterations after small bowel resection in rats. Am J Physiol-Gastrointest Liver Physiol 281(3):G779-G785, 2001
Bines J, Taylor RG, Justice FA, Paris MCJ, Sourial M, Nagy E, Emselle S, Catto-Smith AG, Fuller PJ: The influence of diet complexity on intestinal adaptation following massive small bowel resection in a preclinical model. J Gastro Hepatol 17:1170-1179, 2002
Hartmann B, Johnsen AH, Ørskov C, Adelhorst K, Thim L, Holst JJ: Structure, measurement and secretion of human glucagon-like peptide-2. Peptides 21:73-80, 2000
Barnes GL, Ford RPK, Dawson S, Lawrance S: "Normal" disaccharidase levels in children. Austr Pediatr J 24:31-33, 1988
Verbeke G, Molenberghs G: Linear Mixed Models for Longitudinal Data. New York, Springer Verlag, 2000, pp 55-76
Williamson RCN: Intestinal adaptation. Structural, functional and cytokinetic changes. N Engl J Med 298:1393-1402, 1978
Weser E, Heller R, Tawil T: Stimulation of mucosal growth in the rat ileum by bile and pancreatic secretions after jejunal resection. Gastroenterology 73:524-529, 1977
Wolvekamp MCJ, Durante NMC, Heineman E, Meijssen MAC, Bijman J, de Jonge HR, Marquet RL, Molenaar JC: The value of in vivo electrophysiological measurements in the follow-up of intestinal adaptation after massive small bowel resection in the rat. Gut 34:637-642, 1993
Bury KD: Carbohydrate digestion and absorption after massive resection of the small intestine. Surg Gynecol Obstet 135:177-187, 1972
Justice FA, Wolvekamp MCJ, Taylor RG, Fuller PJ: Response of genes for prostaglandin synthesis and apoptosis to massive small bowel resection. Int J Surg Invest 3(2):135-140, 2001
Taylor RG, Verity K, Fuller PJ: Rat ileal glucagon gene expression: Ontogeny and response to massive small bowel resection. Gastroenterology 99:724-729, 1990
Taylor RG, Beveridge DJ, Nakamura T, Fuller PJ: Hepatocyte growth factor gene expression after massive small bowel resection: Lack of stimulation in lung and liver. Exp Clin Endocrinol 103:58-62, 1995
Rountree DB, Ulshen MH, Selub S, Fuller CR, Bloom SR, Ghatei MA, Lund PK: Nutrient-independent increases in proglucagon and ornithine decarboxylase messenger RNAs after jejunal resection. Gastroenterology 103:462-468, 1992
Karali TT: Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Deposit 16:351-380, 1995
Miller ER, Ullrey DE: The pig as a model for human nutrition. Annu Rev Nutr 7:361-382, 1987
Moughan PJ, Rowan AM: The pig as a model for human nutrition research. Proc Nutr Soc NZ 14:116-123, 1989
Thulesen J, Hartmann B, Kissow H, Jeppesen PB, Orskov C, Holst JJ, Poulsen SS: Intestinal growth adaptation and glucagonlike peptide 2 in rats with ileal-jejunal transposition or small bowel resection. Dig Dis Sci 46(2):379-388, 2001
Burrin DG, Stoll B, Jiang R, Petersen Y, Elnif J, Buddington RK, Schmidt M, Holst JJ, Hartmann B, Sangild PT: GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am J Physiol Gastrointest Liver Physiol 279(6):G1249-G1256, 2000
Dahly EM, Gillingham MB, Guo Z, Murali SG, Nelson DW, Holst JJ, Ney DM: Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. AmJ Physiol Gastr Liver Physiol 284(4):G670-G682, 2003
L'Hereux, Brubaker PL: Therapeutic potential of the intestinotrophic hormone, glucagon-like peptide-2. AnnMed 33(4):229-235, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paris, M.C., Fuller, P.J., Carstensen, B. et al. Plasma GLP-2 Levels and Intestinal Markers in the Juvenile Pig During Intestinal Adaptation: Effects of Different Diet Regimens. Dig Dis Sci 49, 1688–1695 (2004). https://doi.org/10.1023/B:DDAS.0000043388.52260.2f
Issue Date:
DOI: https://doi.org/10.1023/B:DDAS.0000043388.52260.2f