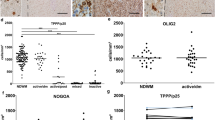
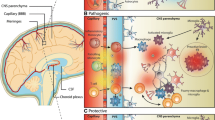
Abstract
Hematogenous macrophages and resident brain microglia are agents of demyelination in multiple sclerosis (MS) and paradoxically may also participate in remyelination. In vitro studies have shown that macrophage enrichment of aggregate brain cultures promotes myelination per se and enhances the capacity to remyelinate following a demyelinating episode. It has been hypothesized that remyelination in MS is implemented by surviving dedifferentiated oligodendrocytes or by newly recruited progenitors that migrate, proliferate and synthesize myelin in response to signalling molecules in the local environment. We postulate that macrophage-derived cytokines or growth factors may directly or indirectly promote oligodendroglial proliferation and differentiation, contributing to myelin repair in inflammatory demyelinating disease.
Similar content being viewed by others
REFERENCES
Nathan, C. F. 1987. Secretory products of macrophages. J. Clin. Invest. 79:319-326
Cuzner, M. L. 1997. Molecular biology of microglia. In: Pages 97-120, Russell, W. C. (ed) Molecular Biology of Multiple Sclerosis. John Wiley & Sons, London.
Prineas, J. W., Barnard, R. O., Kwon, E. E., Sharer, L. R., and Cho, E. S. 1993. Multiple sclerosis: remyelination of nascent lesions. Ann. Neurol. 33:137-151
Loughlin, A. J., Honegger, P., Woodroofe, M. N., Comte, V., Matthieu, J. M., and Cuzner, M. L., 1994. Myelin basic protein content of aggregating rat brain cell cultures treated with cytokines and/or demyelinating antibody: Effects of macrophage enrichement. J. Neurosci. Res. 37:647-653
Loughlin, A. J., Copelman, C. A., Hall, A., Armer, T., Young, B. C., Landon, D. N., and Cuzner, M. L. 1997. Myelination and remyelination of aggregate rat brain cell cultures enriched with macrophages. J. Neurosci. Res. 47:384-392
Hamilton, S. P., and Rome, L. H. 1994. Stimulation of in vitro myelin synthesis by microglia. GLIA 11:326-335
Henderson, B., and Blake, S. 1992. Therapeutic potential of cytokine manipulation. TIPS 13:145-147
Barres, B. A., Raff, M. C., Gaese, F., Bartke, I., Dechant, G., and Barde, Y. A. 1994. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature 367:371-375
Wetmore, C., and Olson, L. 1995. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J. Comp. Neurol. 353:143-159
Cohen, R. I., Marmur, R., Norton, W. T., Mehler, M. F., and Kessler, J. A. 1996. Nerve growth factor and neurotrophin-3 differentially regulate the proliferation and survival of developing rat brain oligodendrocytes. J. Neurosci. 16:6433-6442
Masters, B. A., Werner, H., Roberts, C. T., LeRoith, D., and Raizada, M. K. 1991. Insulin-like growth factor I (IGF-I) receptors and IGF-I action in oligodendrocytes from rat brains. Regul. Pept. 33:117-131
Simpson, D. L., Morrison, R., de-Vellis, J., and Herschman, H. R. 1982. Epidermal growth factor binding and mitogenic activity on purified populations of cells from the central nervous system. J. Neurosci. Res. 8:453-462
Bansal, R., Kumar, M., Murray, K., Morrison, R. S., and Pfeiffer, S. E. 1996. Regulation of FGF receptors in the oligodendrocyte lineage. Mol. Cellul. Neurosci. 7:263-275
Miyake, A., Hattori, Y., Ohta, M., and Itoh, N. 1996. Rat oligodendrocytes and astrocytes preferentially express fibroblast growth factor receptor-2 and-3 mRNAs. J. Neurosci. Res. 45:534-541
Pringle, N. P., Mudhar, H. S., Collarini, E. J., and Richardson, W. D. 1992. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115:535-551
Otero, G. C., and Merrill, J. E. 1994. Cytokine receptors on glial cells. GLIA 11:117-128
Woodroofe, M. N., Bellamy, A. S., Feldmann, M., Davison, A. N., and Cuzner, M. L. 1986. Immunocytochemical characterisation of the immune reaction in the central nervous system in multiple sclerosis. J. Neurol. Sci. 74:135-152
Esiri, M. M., and Reading, M. C. 1987. Macrophage populations associated with multiple sclerosis plaques. Neuropathol. Appl. Neurobiol. 13:451-465
Lampert, P. W. 1965. Demyelination and remyelination in experimental allergic encephalomyelitis, further electron microscopic observations. J. Neuropath. Exp. Neurol. 24:371-385
Prineas, J. W., and Wright, R. G. 1978. Macrophages, lymphocytes, and plasma cells in the perivascular compartments in chronic multiple sclerosis. Lab. Invest. 38:409-421
Brosnan, C. F., Bloom, B. R., and Bornstein, M. B. 1981. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J. Immunol. 126:614-620
Huitinga, I., Van Rooijen, N., deGroot, C. J. A., Uitdehaag, B. M. J., and Dijkstra, C.D. 1990. Elimination of macrophages infiltrating the CNS suppresses EAE in rats. J. Exp. Med. 172:1025-1033
Raine, C. S., Scheinberg, L., and Waltz, J. M. 1981. Multiple sclerosis: oligodendrocyte survival and proliferation in an active, established lesion. Lab. Invest. 45:534-546
Brosnan, C. F., and Raine, C. S. 1996. Mechanisms of immune injury in multiple sclerosis. Brain Pathol. 6:243-257
Giulian, D., Chen, J., Ingeman, J. E., George, J. K., and Noponen, M. 1989. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J. Neurosci. 9:4416-4429
Graca, D. L., and Blakemore, W. F. 1986. Delayed remyelination in rat spinal cord following ethidium bromide injection. Neuropathol. Appl. Neurobiol. 12:593-605
Li, H., Cuzner, M. L., and Newcombe, J. 1996. Microglia-derived macrophages in early multiple sclerosis plaques. Neuropathol. Appl. Neurobiol. 22:207-215
Raivich, G., Moreno-Flores, M. T., Moller, J. C., and Kreutzberg, G. W. 1994. Inhibition of posttraumatic microglial proliferation in a genetic model of macrophage colony-stimulating factor deficiency in the mouse. Eur. J. Neurosci. 6:1615-1618
Raivich, G., Gehrmann, J., and Kreutzberg, G. W. 1991. Increase of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor receptors in the regenerating rat facial nucleus. J. Neurosci. Res. 30:682-686
Lisak, R. P. 1996. In vitro studies of glial cells: what can we learn about demyelinating diseases? Multiple Sclerosis 2:173-178
Heumann, R., Lindholm, D., Bandtlow, C., Meyer, M., Radeke, M. J., Misko, T. P., Shooter, E., and Thoenen, H. 1987. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc. Natl. Acad. Sci. USA 84:8735-8739
Lazarov-Spiegler, O., Solomon, A. S., Ben Zeev-Brann, A., Hirschberg, D. L., Lavie, V., and Schwartz, M. 1996. Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB J. 10:1296-1302
Cross, A. H., Cannella, B., Brosnan, C. F., and Raine, C. S. 1991. Hypothesis: antigen-specific T cells prime central nervous system endothelium for recruitment of nonspecific inflammatory cells to effect autoimmune demyelination. J. Neuroimmunol. 33:237-244
Prineas, J. W., Kwon, E. E., Goldenberg, P. Z., Ilyas, A. A., Quarles, R. H., Benjamins, J. A., and Sprinkle, T. J. 1989. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab. Invest. 61:489-503
Dubois-Dalcq, M., and Armstrong, R. 1990. The cellular and molecular events of central nervous system remyelination. BioEssays 12:569-576
Barres, B. A., Hart, I. K., Coles, H. S., Burne, J. F., Voyvodic, J. T., Richardson, W. D., and Raff, M. C. 1992. Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70:31-46
Noble, M., Murray, K., Stroobant, P., Waterfield, M. D., and Riddle, P. 1988. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333:560-562
Richardson, W. D., Pringle, N., Mosley, M. J., Westermark, B., and Dubois-Dalcq, M. 1988. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell 53:309-319
Honegger, P., and Tenot-Sparti, M. 1992. Developmental effects of basic fibroblast growth factor and platelet-derived growth factor on glial cells in a three-dimensional cell culture system. J. Neuroimmunol. 40:295-304
McKinnon, R. D., Piras, G., Ida, J. A., and Dubois-Dalcq, M. 1993. A role for TGF-Beta in oligodendrocyte differentiation. J. Cell Biol. 121:1397-1407
Besnard, F., Perraud, F., Sensenbrenner, M., and Labourdette, G. 1989. Effects of acidic and basic fibroblast growth factors on proliferation and maturation of cultured rat oligodendrocytes. Int. J. Dev. Neurosci. 7:401-409
McMorris, F. A., and Dubois-Dalcq, M. 1988. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J. Neurosci. Res. 21:199-209
Benveniste, E. N., and Merrill, J. E. 1986. Stimulation of oligodendroglial proliferation and maturation by interleukin-2. Nature 321:610-613
Raff, M. C. 1989. Glial cell diversification in the rat optic nerve. Science 243:1450-1455
Hughes, S. M., Lillien, L. E., Raff, M. C., Rohrer, H., and Sendtner, M. 1988. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature 335:70-73
Barres, B. A., Schmid, R., Sendtner, M., and Raff, M. C. 1993. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 118:283-295
Barres, B. A., Burne, J. F., Holtmann, B., Thoenen, H., Sendtner, M., and Raff, M. C. 1996. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol. Cellul. Neurosci. 8:146-156
Barde, Y. A. 1994. Neurotrophins: a family of proteins supporting the survival of neurons. Prog. Clin. Biol. Res. 390:45-56
Snider, W. D. 1994. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77:627-638
Grinspan, J., Wrabetz, L., and Kamholz, J. 1993. Oligodendrocyte maturation and myelin gene expression in PDGF-treated cultures from rat cerebral white matter. J. Neurocytol. 22:322-333
Honegger, P., and Guentert Lauber, B. 1983. Epidermal growth factor (EGF) stimulation of cultured brain cells. I. Enhancement of the developmental increase in glial enzymatic activity. Brain Res. 313:245-251
Almazan, G., Honegger, P., Matthieu, J. M., and Guentert-Lauber, B. 1985. Epidermal growth factor and bovine growth hormone stimulate differentiation and myelination of brain cell aggregates in culture. Brain Res. 353:257-264
McKay, J. S., Blakemore, W. F., and Franklin, R. J. M. 1997. The effects of the growth factor-antagonist, trapidil, on remyelination in the CNS. Neuropathol. Appl. Neurobiol. 23:50-58
Van der Pal, R. H., Koper, J. W., Van Golde, L. M., and Lopes Cardozo, M. 1988. Effects of insulin and insulin-like growth factor (IGF-I) on oligodendrocyte-enriched glial cultures. J. Neurosci. Res. 19:483-490
Mozell, R. L., and McMorris, F. A. 1991. Insulin-like growth factor I stimulates oligodendrocyte development and myelination in rat brain aggregate cultures. J. Neurosci. Res. 30:382-390
Carson, M. J., Behringer, R. R., Brinster, R. L., and McMorris, F. A. 1993. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 10:729-740
Carson, M. J., Behringer, R. R., Mathews, L. S., Palmiter, R. D., Brinster, R. L., and McMorris, F. A. 1989. Hypomyelination caused by growth hormone deficiency is reversed by insulin-like growth factor 1 in transgenic mice. Trans. Am. Soc. Neurochem. 20:286
Beck, K. D., Powell Braxton, L., Widmer, H. R., Valverde, J., and Hefti, F. 1995. IGF-1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14:717-730
Yao, D. L., Liu, X., Hudson, L. D., and Webster, H. D. 1995. Insulin-like growth factor 1 treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 92:6190-6194
Yao, D. L., West, N. R., Bondy, C. A., Brenner, M., Hudson, L. D., Zhou, J., Collins, G. H., and Webster, H. D. 1995. Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor 1 and insulin-like growth factor binding protein 2 during myelin regeneration. J. Neurosci. Res. 40:647-659
Komoly, S., Hudson, L. D., Webster, H. D., and Bondy, C. A. 1992. Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proc. Natl. Acad. Sci. USA 89:1894-1898
Newcombe, J., Gveric, D., Strand, C., and Cuzner, M. L. 1996. Expression of the insulin-like growth factor cytokine family in multiple sclerosis lesions. Multiple Sclerosis 2:6-7(Abstract)
Fressinaud, C., Vallat, J. M., and Labourdette, G. 1995. Basic fibroblast growth factor down-regulates myelin protein gene expression and alters myelin compaction of mature oligodendrocytes in vitro. J. Neurosci. Res. 40:285-293
Oh, L. Y. S., and Yong, V. W. 1996. Astrocytes promote process outgrowth by adult human oligodendrocytes in vitro through interaction between bFGF and astrocyte extracellular matrix. GLIA 17:237-253
Fressinaud, C., and Vallat, J. M. 1994. Basic fibroblast growth factor improves recovery after chemically induced breakdown of myelin-like membranes in pure oligodendrocyte cultures. J. Neurosci. Res. 38:202-213
Louis, J. C., Magal, E., Takayama, S., and Varon, S. 1993. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science 259:689-692
D'Souza, S. D., Alinauskas, K. A., and Antel, J. P. 1996. Ciliary neurotrophic factor selectively protects human oligodendrocytes from tumor necrosis factor-mediated injury. J. Neurosci. Res. 43:289-298
Armstrong, R. H. L., and Dubois Dalcq, M. 1990. Type-1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate towards distinct molecules. J. Neurosci. Res. 27:400-407
Araujo, D. M., and Cotman, C. W. 1992. Basic FGF in astroglial, microglial, and neuronal cultures: characterization of binding sites and modulation of release by lymphokines and trophic factors. J. Neurosci. 12:1668-1678
Bonner, J. C., Osornio-Vargas, A. R., Badgett, A., and Brody, A. R. 1991. Differential proliferation of rat lung fibrolasts induced by the platelet-derived growth factor-AA,-AB, and-BB isoforms secreted by rat alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 5:539-547
Plata-Salaman, C. R. 1991. Epidermal growth factor and the nervous system. Peptides 12:653-663
Nagaoka, I., Someya, A., Iwabuchi, K., and Yamashita, T. 1991. Expression of insulin-like growth factor-1A and factor 1B mRNA in human liver, hepatoma cells, macrophage-like cells and fibrolasts. FEBS. Lett. 280:79-83
Merrill, J. E., Ignarro, L. J., Sherman, M. P., Melinek, J., and Lane, T. E. 1993. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J. Immunol. 15:2132-2141
Elkabes, S., DiCicco-Bloom, E. M., and Black, I. B. 1996. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 16:2508-2521
Ip, N. Y., Wiegand, S. J., Morse, J., and Rudge, J. S. 1993. Injury-induced regulation of ciliary neurotrophic factor mRNA in the adult rat brain. Eur. J. Neurosci. 5:25-33
Gilad, G. M., and Gilad, V. H. 1995. Chemotaxis and accumulation of nerve growth factor by microglia and macrophages. J. Neurosci. Res. 41:594-602
Maisonpierre, P. C., Belluscio, L., Friedman, B., Alderson, R. A., Wiegand, S. J., Furth, M. E., Lindsay, R. A., and Yancopoulos, G. D. 1990. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5:509-509
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Diemel, L., Copelman, C. & Cuzner, M. Macrophages in CNS Remyelination: Friend or Foe?. Neurochem Res 23, 341–347 (1998). https://doi.org/10.1023/A:1022405516630
Issue Date:
DOI: https://doi.org/10.1023/A:1022405516630