Abstract
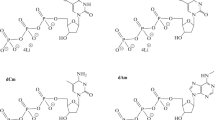
The dependence of the modification efficiency of DNA polymerases and DNA template on the nature of photoreactive group and the length of the linker that joins the group with the heterocyclic base of the primer 3"-terminal nucleotide was studied. The primers that contained the photoreactive groups at their 3"-termini were obtained using the rat DNA polymerase β or the DNA polymerase from Thermus thermophilus in the presence of one of the dTTP analogues carrying the photoreactive group in position 5 of thymidine residue. After irradiating the reaction mixture with UV light and separating the modification products, the level of covalent attachment of the [5"-32P]primer to DNA polymerases and template was determined. The primers containing 4-azido-2,5-difluoro-3-chloropyridyl group were shown to be the most effective in the modification of DNA polymerases.
Similar content being viewed by others
References
Knorre, D.G., Kudryashova, I.V., and Lavrik, O.I., Usp. Khim., 1998, vol. 67, pp. 486–502.
Wlassoff, W.A., Dobrikov, M.I., Safronov, I.V., Dudko, R.Y., Bogachev, V.S., Kandaurova, V.V., Shishkin, G.V., Dymshits, G.M., and Lavrik, O.I., Bioconjugate Chem., 1995, vol. 6, pp. 352–360.
Kolpashchikov, D.M., Zakharenko, A.L., Dezhurov, S.V., Rechkunova, N.I., Khodyreva, S.N., Degtyarev, S.Kh., Litvak, V.V., and Lavrik, O.I., Bioorg. Khim., 1999, vol. 25, pp. 129–136.
Kolpashchikov, D.M., Ivanova, T.M., Bogachev, V.S., Nasheuer, H.-P., Weisshart, K., Favre, A., and Lavrik, O.I., Bioconjugate Chem., 2000, vol. 11, pp. 445–451.
Godovikova, T.S., Kolpashchikov, D.M., Orlova, T.N., Richter, V.A., Ivanova, T.M., Grochovsky, S.L., Nasedkina, T.V., Victorova, L.S., and Poletaev, A.I., Bioconjugate Chem., 1999, vol. 10, pp. 529–537.
Doronin, S.V., Dobrikov, M.I., Buckle, M., Roux, P., Buc, H., and Lavrik, O.I., FEBS Lett., 1994, vol. 354, pp. 200–202.
Safronov, I.V., Shcherbik, N.V., Khodyreva, S.N., Vlasov, V.A., Dobrikov, M.I., Shishkin, G.V., and Lavrik, O.I., Bioorg. Khim., 1997, vol. 23, pp. 576–585.
Doronin, S.V., Dobrikov, M.I., and Lavrik, O.I., FEBS Lett., 1992, vol. 313, pp. 31–33.
Lavrik, O.I., Nasheuer, H.P., Weisshart, K., Wold, M.S., Prasad, R., Beard, W.A., Wilson, S.H., and Favre, A., Nucleic Acids Res., 1998, vol. 26, pp. 602–607.
Dobrikov, M.I., Gorn, V.V., Zarytova, V.F., Levina, A.S., Prikhod'ko, T.A., Shishkin, G.V., Tabatadze, D.R., and Zaalishvili, M.M., Bioorg. Khim., 1992, vol. 18, pp. 1190–1198.
Dobrikov, M.I., Zarytova, V.F., Komarova, N.I., Levina, A.S., Lokhov, S.A., Prikhod'ko, T.A., Shishkin, G.V., Tabatadze, D.R., and Zaalishvili, M.M., Bioorg. Khim., 1992, vol. 18, pp. 540–549.
Liu, M.T., Chem. Soc. Rev., 1981, vol. 11, p. 127.
Yamaguchi, T. and Saneyoshi, M., Nucleic Acids Res., 1996, vol. 17, pp. 3364–3369.
Drachkova, I.A., Petruseva, I.O., Safronov, I.V., Zakharenko, A.L., Shishkin, G.V., Lavrik, O.I., and Khodyreva, S.N., Bioorg. Khim., 2001, vol. 27, pp. 197–204.
Yakubov, L., Khald, Z., Zhang, L.-M., Truneh, A., Vlassov, V., and Stein, C.A., J. Biol. Chem., 1993, vol. 398, pp. 18 818–18 823.
Khlimankov, D.Yu., Petruseva, I.O., Rechkunova, N.I., Belousova, E.A., Kolpashchikov, D.M., Khodyreva, S.N., and Lavrik, O.I., Bioorg. Khim., 2001, vol. 27, pp. 205–209.
Beard, W.A. and Wilson, S.H., Methods Enzymol., 1995, vol. 262, pp. 98–107.
Rechkunova, N.I., Akishev, A.G., Lebedeva, N.A., Khodyreva, S.N., Degtyarev, S.Kh., and Lavrik, O.I., Biokhimiya (Moscow), 1998, vol. 63, pp. 1490–1495.
Langer, P.R., Waldrop, A.A., and Ward, D.C., Proc. Natl. Acad. Sci. USA, 1981, vol. 78, pp. 6633–6637.
Yamshchikov, V.F., Metody molekulyarnoi genetiki i gennoi inzhenerii (Methods of Molecular Genetics and Genetic Engineering), Salganik, R.I., Ed., Moscow: Nauka, 1990, pp. 112–114.
Sambrook, J., Fritsch, E.F., and Maniatis, T., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, New York: Cold Spring Harbor Lab., 1989.
Laemmli, U.K., Nature, 1970, vol. 277, pp. 680–685.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dezhurov, S.V., Khodyreva, S.N., Rechkunova, N.I. et al. A Comparative Study of the Modification Efficiency of DNA Polymerases and DNA Template by the DNA Primers with Various Photoreactive Groups at Their 3"-Termini. Russian Journal of Bioorganic Chemistry 29, 66–72 (2003). https://doi.org/10.1023/A:1022234620315
Issue Date:
DOI: https://doi.org/10.1023/A:1022234620315