Abstract
Inflammation is a primary reaction to infection, allergic disorders, autoimmune diseases, and mechanical injury. The goal of an inflammatory response is to rapidly respond to noxious stimuli, such as trauma or pathogen, with a controlled amplification of cellular activation to eliminate, control, or wall off the triggering agent. Although the inflammatory response is necessary for resolution of the pathogenic event, by stander or collateral tissue damage is caused by the toxic nature of many of its by-products. It is characterized by the infiltration of leukocytes into the affected area. Chemokines and their receptors play an essential role as mediators of leukocyte infiltration. In most cases this response is so vigorous that its control, especially in the central nervous system, would inhibit recovery. The benefits of anti-inflammatory therapy based on interference with the chemokine system has been established in animal models and is being pursued with chemokine antibodies and receptor antagonists. Prolonged treatment with a broad-spectrum chemokine antagonist, vMIPII, has been shown to reduce the rate of infiltration of monocytes into injured rat spinal cord and promote survival.
Similar content being viewed by others
REFERENCES
Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature 392:565–568.
Howard, O. M. Z., Oppenheim, J. J., and Wang, J. M. 1999. Chemokines as molecular targets for therapeutic intervention. J. Clinical Immunol. 19:280–292.
Huang, D., Han, Y., Sandhya Rani, M. R., Glabinski, A., Trebst, C., Sorensen, T., Tani, M., Wang, J., Chien, P., O'Bryan, S., Bielecki, B., Zhou, Z. L., Majumder, S., and Ransohoff, R. M. 2000. Chemokines and chemokine receptors in inflammation of the nervous system: Manifold roles and exquisite regulation. Immunol. Rev. 177:52–67.
Proudfoot, A. E. I., Power, C. A., and Wells, T. N. C. 2000. The strategy of blocking the chemokine system to combat disease. Immunol. Rev. 177:246–256.
Anthony, D. C., Blond, D., Dempster, R., and Perry, V. H. 2001. Chemokine targets in acute brain injury and disease. Prog. Brain Res. 132:507–524.
Baggiolini, M. 2001. Chemokines in pathology and medicine. J. Intern. Med. 250:91–104.
Bajetto, A., Bonavia, R., Barbero, S., Florio, T., and Schettini, G. 2001. Chemokines and their receptors in the central nervous system. Frontiers Neuroendocrinol. 22:147–184.
DeGroot, C. J. A. and Woodroofe, M. N. 2001. The role of chemokine receptors in CNS inflammation. Progr. in Brain Res. 132:533–544.
Gerard, C. and Rollins, B. J. 2001. Chemokines and disease. Nature Immunol. 2:108–115.
Horuk, R. 2001. Survey chemokine receptors. Cytokine Growth Factor Rev. 12:313–335.
Mackay, C. R. 2001. Chemokines: Immunology's high impact factors. Nature Immunol. 2:95–101.
Strieter, M., Standiford, T. J., Huffnagle, G. B., Colletti, L. M., Lukacs, N. W., and Kunkel, S. L. 1996. “The good, the bad, and the ugly”: The role of chemokines in models of human disease. J. Immunol. 156:3583–3586.
Sekido, N., Mukaida, N., Harada, A., Nakanishi, X., Watanabe, Y., and Matsushima, K. 1993. Prevention of lung perfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature 365:654–657.
Nishimura, A., Akahoshi, T., Takahashi, M., Takagishi, K., Itoman, M., Kondo, H., Takahashi, Y., Mukaida, N., and Matsushima, K. 1997. Attenuation of monosodium urate crystal-induced arthritis in rabbits by a neutralizing antibody against interleukin-8. J. Leukocyte Biol. 62:444–449.
Feng, L., Xia, Y., Yoshimura, T., and Wilson C. B. 1995. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J. Clin. Invest. 95:1009–1017.
Rand, M. L., Warren, J. S., Mansour, M. K., Newman, W., and Ringler, D. J. 1996. Inhibition of T cell recruitment and cutaneous delayed-type hypersensitivity-induced inflammation with antibodies to monocyte chemoattractant protein-1. Am. J. Pathol. 148:855–864.
Lukacs, N. W., Streiter, R. M., Warmington, K., Lincoln, P., Chensue, S. W., and Kunkel, S. L. 1997. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J. Immunol. 158:4398–4404.
Gong, J. H., Ratkay, L. G., Waterfield, J. D., and Clark-Lewis, I. 1997. An antagonist of monocyte chemoattractant protein-1 (MCP-1) inhibits arthritis in the MRL-1pr mouse model. J. Exp. Med. 186:131–137.
Plater-Zyberk, C., Hoogeewerf, A. J., Proudfoot, A. E. I., Power, C. A., and Wells, T. N. C. 1977. Effect of CC chemokine receptor antagonist on collagen induced arthritis in DBA/I mice. Immunol. Lett. 57:117–120.
Zhou, N., Luo, Z., Hall, J. W., and Huang, Z. 2000. A novel peptide antagonist of CXCR4 derived from the N-terminus of viral chemokine vMIPII. Biochemistry 39:3782–3787.
Gao, J. L. and Murphy, P. M. 1994. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J. Biol. Chem. 269:28539–28542.
Ahuja, S. K. and Murphy, P. M. 1993. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J. Biol. Chem. 268:20691–20694.
Ahuja, S. K., Gao, J. L., and Murphy, P. M. 1994. Chemokine receptor and molecular mimicry. Immunol. Today 15:281–287.
Murayama, T., Kuno, K., Jisaki, F., Obuchi, M., Sakamuro, D., Furukawa, T., Mukaida, N., and Matsushima, K. (1994) Enhancement human cytogalovirus replication in a human lung fibroblast cell line by interleukin-8. J. Virol. 68:7582–7585.
Alcami, A., Symons, J. A., Collins, P. D., Williams, T. J., and Smith, G. L. 1998. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 160:624–633.
Anderson, D. K. 1992. Chemical and cellular mediators in spinal cord injury. J. Neurotrauma. 9:143–145; discussion 145–146.
Faden, A. I. 1996. Pharmacological treatment of central nervous system trauma. Pharmacol. Toxicol. 78:12–7.
Martiney, J. A., Cuff, C., Litwak, M., Berman, J., and Brosnan, C. F. 1998. Cytokine-induced inflammation in the central nervous system revisited. Neurochem. Res. 23:349–359.
Hayashi, M., Ueyama, T., Tamaki, T., and Senba, E. 1997. Expression of neurotrophin and IL-1 beta mRNAs following spinal cord injury and the effects of methylprednisolone treatment. Kaibogaku Zasshi. J. Anat. 72:209–213.
Ichitani, Y., Shi, T., Haeggstrom, J. Z., Samuelsson, B., and Hokfelt, T. 1997. Increased levels of cyclooxygenase-2 mRNA in the rat spinal cord after peripheral inflammation: An in situ hybridization study. Neuroreport 8(13):2949–2952.
Yong, V. W. 1996. Cytokines: Astrogliosis and neurotrophism following CNS trauma. Page 309, In Cytokines and the CNS, R. M. Ransohoff and E. N. Benveniste (eds), CRC Press Inc. Boca Raton, Florida.
Eng, L. F., Ghirnikar, R. S., and Lee, Y. L. 1996. Inflammation in EAE: Role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem. Res. 21:511–525.
Ghirnikar, R. S., Lee, Y. L., and Eng, L. F. 1998. Inflammation in traumatic brain injury: Role of cytokines and chemokines. Neurochem. Res. 23:329–340.
Dong, Y. and Benveniste, E. N. 2001. Immune function of astrocytes. Glia. 36:180–190.
Hausmann, E. H. S., Berman, N. E. J., Wang, Y.-Y., Meara, J. B., Wood, G. W., and Klein, R. M. 1998. Selective chemokine mRNA expression following brain injury. Brain Res. 788:49–59.
Baggiolini, M. 2000. Reflection on chemokines. Immunol. Rev. 177:5–7.
Boring, L., Gosling, J., Chensue, S. W., Kunkel, S. L., Farese, R. V., Jr., Broxmeyer, H. E., and Charo, I. F. 1997. Impaired monocyte migration and reduced type 1 (Th1) cytokine response in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 100:2552–2561.
Boring, L., Gosling, J., Cleary, M., and Charo, I. F. 1998. Decreased lesion formation in CCR2-/-mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394: 894–897.
Kurihara, T., Warr, G., Loy, J., and Bravo, R. 1997. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186:1757–1762.
Kuziel, W. A., Morgan, S. J., Dawson, T. C., Griffin, S., Smithies, O., Ley, K., and Maeda, N. 1997. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Nat. Acad. Sci. U.S.A. 94:12053–12058.
Fife, B. T., Huffnagle, G. B., Kuziel, W. A., and Karpus, W. J. 2000. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 192: 899–905.
Izikson, L., Klein, R. S., Charo, I. F., Weiner, H. L., and Luster, A. D. 2000. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192:1075–1080.
Ghirnikar, R. S., Lee, Y. L., LiJ. D., and Eng, L. F. 1998. Chemokine inhibition in rat stab wound brain injury using antisense oligodeoxynucleotides. Neurosci. Lttr. 247:21–14.
Popovich P., Wei P., Stokes B. T. 1997. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 377:443–464.
McTigue, D. M., Tani, M., Krivacic, K., Chernosky, A., Kelner, G. S., Maciejewski, D., Maki, R., Ransohoff, R. M., and Stokes, B. T. 1998. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J. Neurosci. Res. 53:368–376.
Lee, Y. L., Shih, K., Bao, P., Ghirnikar, R. S., and Eng, L. F. 2000. Cytokine/chemokine expression in contused rat spinal cord. Neurochem. Intl. 36:417–425.
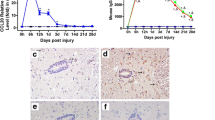
Ghirnikar, R. S., Lee, Y. L., and Eng, L. F. 2000. Chemokine antagonist infusion attenuates cellular infiltration following contusion injury in rat. J. Neurosci. Res. 59:63–73.
Ghirnikar, R. S., Lee, Y. L., and Eng, L. F. 2001. Chemokine antagonist infusion promotes axonal sparing following spinal cord contiusion injury in rat. J. Neurosci. Res. 64:582–589.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eng, L.F., Lee, Y.L. Response of Chemokine Antagonists to Inflammation in Injured Spinal Cord. Neurochem Res 28, 95–100 (2003). https://doi.org/10.1023/A:1021652229667
Issue Date:
DOI: https://doi.org/10.1023/A:1021652229667