Abstract
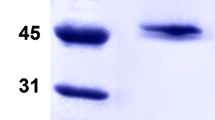
An α-L-fucosidase (E.C. 3.2.1.51) exhibiting a wide aglycon specificity expressed in ability of cleaving α1 → 6-, α1 →3-, α1 → 4-, and α1 → 2-O-fucosyl bonds in fucosylated oligosaccharides, has been isolated from culture filtrate of Thermus sp. strain Y5. The α-L-fucosidase hydrolyzes p-nitrophenyl α-L-fucopyranoside with V max of 12.0 ± 0.1 μM/min/mg and K m = 0.20 ± 0.05 mM and is able to cleave off about 90% of total L-fucose from pronase-treated fractions of fucosyl-containing glycoproteins and about 30% from the native glycoproteins. The purified enzyme is a tetramer with a molecular mass of 240 ± 10 kDa consisting of four identical subunits with a molecular mass of 61.0 ± 0.5 kDa. The N-terminal sequence showed homology to some α-L-fucosidases from microbial and plant sources. Hydrolysis of p-nitrophenyl α-L-fucopyranoside occurs with retention of the anomeric configuration. Transglycosylating activity of the α-L-fucosidase was demonstrated in reactions with such acceptors as alcohols, N-acetylglucosamine and N-acetylgalactosamine while no transglycosylation products were observed in the reaction with p-nitrophenyl α-L-fucopyranoside. The enzyme can be classified in glycosyl hydrolase family 29.
Similar content being viewed by others
References
Tsuiji Y, Yamamoto K, Tochikura T, Purification and some properties of a novel α-L-fucosidase capable of acting on α-(1→6)-Lfucosidic linkages from Bacillus circulans M28, J Biochem 108, 235–40 (1990).
Sano M, Hayakawa K, Kato I, Purification and characterization of α-L-fucosidase from Streptomyces species, J Biol Chem 267, 1522–7 (1992).
Scudder P, Neville DCA, Butters TD, Fleet GWJ, Dwek RA, Rademacher TW, Jacob GS, The isolation by ligand affinity chromatography of a novel form of α-L-fucosidase from almond, J Biol Chem 265, 16472–7 (1990).
Codina A, Vilaseca M, Tarrago T, Fernandez I, Ludevid D, Giralt E, Location of disulfide bonds in mature α-L-fucosidase from pea, J Pept Sci 7, 305–15 (2001).
Abascal I, Skalaban SR, Grimm KM, Aviles M, Martianez-Menarg JA, Castells MT, Ballesta J, Alhadeff JA, Alteration of the isoform composition of plasma-membrane-associated rat sperm α-L-fucosilate epididymal maturation: Comparative characterization acidic and neutral isoforms, Biochem J 333, 201–7 (1998).
Alhadeff JA, Khunsook S, Choowongkomon K, Baney T, Heredia VA, Bean B, Characterization of human semen α-L-fucosidases, Mol Hum Reprod 5, 809–15 (1999).
DiCioccio RA, Barlow JJ, Matta KL, Substrate specificity and other properties of alpha-L-fucosidase from human serum, J Biol Chem 257, 714–8 (1982).
Aviles M, Abascal I, Martinez-Menarguez JA, Castells MT, Skalaban SR, Ballesta J, Alhadeff JA, Immunocytochemical localization and biochemical characterization of a novel plasma membrane-associated, neutral pH optimal α-L-fucosidase from rat testis and epididymal spermatozoa, Biochem J 318, 821–31 (1996).
Aminoff D, Furukawa K, Enzymes that destroy blood group specificity. I. Purification and properties of α-L-fucosidase from Clostridium perfringens, J Biol Chem 245, 1659–69 (1970).
Bahl OP, Glycosidases of Aspergillus niger. I. Purification and general properties of 1,2-α-L-fucosidase, J Biol Chem 245, 299–304 (1970).
Fukuda M, Molecular Glycobiology (Oxford University Press, Oxford, 1994), pp. 1–52.
Watkins WM, Biochemistry and Genetics of the ABO, Lewis, and P blood group systems, Adv Hum Genet 10, 1–136 (1980).
Springer TA, Lasky LA, Cell adhesion. Sticky sugars for selectins, Nature 349, 196–7 (1991).
Wiese TJ, Dunlap JA, Yorek MA, Effect of L-fucose and D-glucose concentration on L-fucoprotein metabolism in human Hep G2 cells and changes in fucosyltransferase and α-L-fucosidase activity in liver of diabetic rats, Biochim Biophys Acta 1335, 61–72 (1997).
Goi G, Bairati C, Segalini G, Burlina AB, Massaccesi L, Lovagnini A, Lombardo A, Alterations in the activity of several glycohydrolases in red blood cell membrane from type 2 diabetes mellitus patients, Metabolism 48, 817–21 (1999).
Ayude D, Fernandez-Rodriguez J, Rodriguez-Berrocal FJ, Martinez VS, de Carlos A, Gil E, Paez de La Cadena M, Value of the serum α-L-fucosidase activity in the diagnosis of colorectal cancer, Oncology 59, 310–6 (2000).
Inui K, Akagi M, Nishigaki T, Muramatsu T, Tsukamoto H, Okada S, A case of chronic infantile type of fucosidosis: Clinical and magnetic resonance image findings, Brain Dev 22, 47–9 (2000).
Willems PJ, Seo HC, Coucke P, Tonlorenzi R, O'Brien JS, Spectrum of mutations in fucosidosis, Eur J Hum Genet 7, 60–7 (1999).
Fernandez-Rodriguez J, Ayude D, de la Cadena MP, Martinez-Zorzano VS, de Carlos A, Caride-Castro A, de Castro G, Rodriguez-Berrocal FJ, α-L-Fucosidase enzyme in the prediction of colorectal cancer patients at high risk of tumor recurrence, Cancer Detect Trev 24, 143–9 (2000).
Sanchez-Martin MM, Cabezas JA, Ortega S, Garcia J, Garcia-Criado FJ, Pina J, Gomez-Alonso A, Levels of serum cathepsin L and several glycosidases in patients operated for colorectal cancer, Cancer Lett 141, 73–7 (1999).
Shepherd VL, Lee YC, Schlesinger PH, Stahl PD, L-Fucoseterminated glycoconjugates are recognized by pinocytosis receptors on macrophages, Proc Natl Acad Sci USA 78, 1019–22 (1981).
Duus JØ, Nifant'ev NE, Shashkov AS, Khatuntseva EA, Bock K, Synthesis and structural studies of branched 2-linked trisaccharides related to H type 2 blood groop determinants, Izr J Chem 40, 223–39 (2000).
Nifant'ev NE, Amochaeva VY, Shashkov AS, Kochetkov NK, Synthesis, NMR, and conformational studies of branched oligosaccharides. 9. α-Fucosylation by 2,3,4-tri-O-benzoyl-α-Lfucopyranosyl bromide under Helferich conditions, Carbohydr Res 242, 77–91 (1993).
Schachter H, Sarney J, McGuire EJ, Roseman S, Isolation of diphosphopyridine nucleotide-dependent L-fucose dehydrogenase from pork liver, J Biol Chem 244, 4785–92 (1969).
Laemmli UK, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature 227, 680–5 (1970).
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ, Protein measurements with the Folin phenol reagent, J Biol Chem 193, 265–75 (1951).
Alhadeff JA, Miller AL, Wenaas H, Vedvick T, O'Brien JS, Human liver α-L-fucosidase, J Biol Chem 250, 7106–13 (1975).
Tsay GC, Dawson G, A sensitive spectrophotometric method for detection of L-fucose, Anal Biochem 78, 423–7 (1977).
Leatherbarrow RT, Enzifitter (Elsevier Biosoft, Cambridge, 1987), p. 1.
Dixon M, The termination of enzyme inhibitory constant, Biochem J 55, 170–1 (1953).
Eneyskaya EV, Golubev AM, Kachurin AM, Savel'ev AN, Neustroev KN, Transglycosylation activity of α-galactosidase from Trichoderma reesei. An investigation of the active site, Carbohydr Res 305, 83–91 (1998).
Zinin AV, Shabalin KA, Kulminskaya AA, Shishlyannikov SM, Neustroev KN, 1-O-Acetyl-beta-D-galactopyranose: A novel substrate for the transglycosylation reaction catalyzed by the beta-galactosidase from Penicillium sp., Carbohydr Res 337, 635–42 (2002).
Vetere A, Galateo C, Paoletti S, All-aqueous, regiospecific transglycosylation synthesis of 3-O-α-L-fucopyranosyl-2-acetamido-2-deoxy-D-glucopyranose, a building block for the synthesis of branched oligosaccharides, Biochem Biophys Res Commun 234, 358–61 (1997).
Shcherbakova OG, Filatov MV, Camptothecin enhances random integration of transfected DNA into the genome of mammalian cells, Biochim Biophys Acta 1495, 1–3 (2000).
Păhlsson P, Spitalnik SL, The role of glycosylation in synthesis and secretion of β-amyloid precursor protein by Chines hamster ovary cells, Arch Biochem Biophys 331, 177–86 (1996).
Eneyskaya EV, Kulminskaya AA, Savel'ev AN, Shabalin KA, Golubev AM, Neustroev KN, α-Mannosidase from Trichoderma reesei participates in the postsecretory deglycosylation of glycoproteins, Biochem Biophys Res Commun 245, 43–9 (1998).
Bagiyan FG, Eneyskaya EV, Kulminskaya AA, Savel'ev AN, Shabalin KA, Neustroev KN, The action of α-mannosidase from Oerskovia sp. on the mannose-rich O-linked sugar chains of glycoproteins, Eur J Biochem 249, 286–92 (1997).
Savel'ev AN, Eneyskaya EV, Isaeva-Ivanova LS, Shabalin KA, Golubev AM, Neustroev KN, The carbohydrate moiety of α-galactosidase from Trichoderma reesei, Glycoconj. J., 14, 897–905 (1997).
Thompson JD, Higgins DG, Gibson TJ, Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice, Nucleic Acids Research 22, 4673–80 (1994).
Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer EL, The Pfam Protein Families Database, Nucleic Acids Research 30, 276–80 (2002).
Spellman MW, Leonard CK, Basa LJ, Gelineo I, van Halbeek H, Carbohydrate structures of recombinnt soluble human CD4 expressed in Chinese hamster ovary cells, Biochemistry 30, 2395–406 (1991).
Aeed PA, Guido DM, Mathews WR, Elhamer AP, Characterization of the oligosaccaride structures on recombinnt human prorenin expressed in Chinese hamster ovary cells, Biochemistry 31, 6951–61 (1992).
Bergwerff AA, van Oostrum J, Asselberg FA, Burgi R, Hokke CH, Kamerling JP, Vliegenthart JF, Primary structure of N-linked carbohydrate chains of a human chimeric plasminogen activator K2tu-PA expressed in Chinese hamster ovary cells, Eur J Biochem 212, 639–56 (1993).
Withers SG, Aebersold R, Approaches to labeling and identification of active site residues in glycosidases, Protein Sci 4, 361–72 (1995).
Henrissat B, A classification of glycosyl hydrolases based on amino acid sequence similarities, Biochem J 280, 309–16 (1991).
Ajisaka K, Fijimoto H, Miyasato M, An α-L-fucosidase from Penicillium multicolor as a candidate enzyme for the synthesis of α(1→3)-linked fucosyl oligosaccharides by transglycosylation, Carbohydr Res 309, 125–9 (1998).
Opheim DJ, Touster O, The purification and characterization of rat liver lysosomal α-L-fucosidase, J Biol Chem 252, 739–43 (1977).
Carlsen RB, Pierce JG Purification and properties of an α-Lfucosidase from rat epididymis, J Biol Chem 247, 23–32 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eneyskaya, E.V., Kulminskaya, A.A., Kalkkinen, N. et al. An α-L-fucosidase from Thermus sp. with unusually broad specificity. Glycoconj J 18, 827–834 (2001). https://doi.org/10.1023/A:1021163720282
Issue Date:
DOI: https://doi.org/10.1023/A:1021163720282