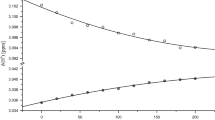
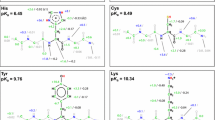
Abstract
α-Lytic protease, a bacterial serine protease of 198 aminoacids (19800 Da), has been used as a model system for studies of catalyticmechanism, structure–function relationships, and more recently forstudies of pro region-assisted protein folding. We have assigned thebackbones of the enzyme alone, and of its complex with the tetrahedraltransition state mimic N-tert-butyloxycarbonyl-Ala-Pro-boroVal, usingdouble- and triple-resonance 3D NMR spectroscopy on uniformly15N- and 13C/15N-labeled protein.Changes in backbone chemical shifts between the uncomplexed and inhibitedform of the protein are correlated with distance from the inhibitor, thedisplacement of backbone nitrogens, and change in hydrogen bond strengthupon inhibitor binding (derived from previously solved crystal structures).A comparison of the solution secondary structure of the uninhibited enzymewith that of the X-ray structure reveals no significant differences.Significant line broadening, indicating intermediate chemical exchange, wasobserved in many of the active site amides (including three broadened toinvisibility), and in a majority of cases the broadening was reversed uponaddition of the inhibitor. Implications and possible mechanisms of this linebroadening are discussed.
Similar content being viewed by others
References
Baker, D., Sohl, J.L. and Agard, D.A. (1992) Nature, 356, 263–265.
Bax, A. and Ikura, M. (1991) J. Biomol. NMR, 1, 99–104.
Bone, R., Shenvi, A.B., Kettner, C.A. and Agard, D.A. (1987) Biochemistry, 26, 7609–7614.
Bone, R., Frank, D., Kettner, C.A. and Agard, D.A. (1989a) Biochemistry, 28, 7600–7609.
Bone, R., Silen, J.L. and Agard, D.A. (1989b) Nature, 339, 191–195.
Bone, R., Fujishige, A., Kettner, C.A. and Agard, D.A. (1991a) Biochemistry, 30, 10388–10398.
Bone, R., Sampson, N.S., Bartlett, P.A. and Agard, D.A. (1991b) Biochemistry, 30, 2263–2272.
Boucher, W., Laue, E.D., Campbell-Burk, S. and Domaille, P.J. (1992a) J. Am. Chem. Soc., 114, 2262–2264.
Boucher, W., Laue, E.D., Campbell-Burk, S. and Domaille, P.J. (1992b) J. Biomol. NMR, 2, 631–637.
Brayer, G.D., Delbaere, L.T.J. and James, M.N.G. (1979) J. Mol. Biol., 131, 743–775.
Clubb, R.T., Thanabal, V. and Wagner, G. (1992) J. Biomol. NMR, 2, 203–210.
Davis, J.H. (1995) J. Biomol. NMR, 5, 433–437.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Dolgikh, D.A., Gilmanshin, R.I., Brazhnikov, E.V., Bychkova, V.E., Semisotnov, G.V., Venyaminov, S.Yu. and Ptitsyn, O.B. (1981) FEBS Lett., 136, 311–315.
Fujinaga, M., Delbaere, L.T.J., Brayer, G.D. and James, M.N.G. (1985) J. Mol. Biol., 184, 479–502.
Grzesiek, S. and Bax, A. (1992) J. Am. Chem. Soc., 114, 6291–6293.
Haggett, K.D., Graham, L.D. and Whitaker, R.G. (1994) Biotechnol. Techniques, 8, 203–208.
Hunkapiller, M.W., Smallcombe, S.H., Whitaker, D.R. and Richards, J.H. (1973) Biochemistry, 12, 4732–4743.
Ikura, M., Kay, L.E. and Bax, A. (1990) Biochemistry, 29, 4659–4667.
Kabsch, W. and Sander, C. (1983) Biopolymers, 12, 2577–2637.
Kay, L.E., Ikura, M., Tschudin, R. and Bax, A. (1990) J. Magn. Reson., 89, 496–514.
Kettner, C.A. and Shenvi, A.B. (1984) J. Biol. Chem., 259, 15106–15114.
Kettner, C.A., Bone, R., Agard, D.A. and Bachovchin, W.W. (1988) Biochemistry, 27, 7682–7688.
Kraulis, P.J. (1989) J. Magn. Reson., 84, 627–633.
Kraulis, P.J., Domaille, P.J., Campbell-Burk, S.L., Vanaken, T. and Laue, E.D. (1994) Biochemistry, 33, 3515–3531.
Levy, G.C. and Lichter, R.L. (1979) Nitrogen-15 Nuclear Magnetic Resonance Spectroscopy, Wiley, New York, NY, U.S.A.
Mace, J.E. and Agard, D.A. (1995) J. Mol. Biol., 254, 720–736.
Mace, J.E., Wilk, B.J. and Agard, D.A. (1995) J. Mol. Biol., 251, 116–134.
Marion, D., Driscoll, P.D., Kay, L.E., Wingfield, P.T., Bax, A., Gronenborn, A.M. and Clore, G.M. (1989a) Biochemistry, 28, 6150–6156.
Marion, D., Kay, L.E., Sparks, S.W., Torchia, D.A. and Bax, A. (1989b) J. Am. Chem. Soc., 111, 1515–1517.
Muhandiram, D.R. and Kay, L.E. (1994) J. Magn. Reson., B103, 203–216.
Silen, J.L., McGrath, C.N., Smith, K.R. and Agard, D.A. (1988) Gene, 69, 237–244.
Silen, J.L., Frank, D., Fujishige, A., Bone, R. and Agard, D.A. (1989) J. Bacteriol., 171, 1320–1325.
Wishart, D.S., Sykes, B.D. and Richards, F.M. (1991) J. Mol. Biol., 222, 311–333.
Wittekind, M., Metzler, W.J. and Müller, L. (1993) J. Magn. Reson., B101, 214–217.
Wittekind, M. and Müller, L. (1993) J. Magn. Reson., B101, 201–205.
Zuiderweg, E.R.P. and Fesik, S.W. (1989) Biochemistry, 28, 2387–2391.
Author information
Authors and Affiliations
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Davis, J.H., Agard, D.A., Handel, T.M. et al. Alterations in chemical shifts and exchange broadening upon peptide boronic acid inhibitor binding to α-lytic protease. J Biomol NMR 10, 21–27 (1997). https://doi.org/10.1023/A:1018314808361
Issue Date:
DOI: https://doi.org/10.1023/A:1018314808361