Abstract
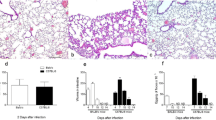
The present study was undertaken to elucidate the participation of Th1 and Th2 responses in granulomatous inflammation induced by Cryptococcus neoformans using Lewis and Brown Norway rats which have Th1-polarized and Th2-polarized innate immunity, respectively. Both strains demonstrated granulomatous inflammation in the lung, and the lesions were composed mainly of mononuclear phagocytes and surrounded by lymphocytes. Lewis rats demonstrated tuberculoid epithelioid cell granulomas with a central necrosis resembling caseation, and increased transcription of Th1 relevant cytokines. On the other hand, Brown Norway rats showed mature granulomas including eosinophils with increased transcription of IL-12 without increased transcription of not only IFN-γ and IL-2 but also Th2 cytokines such as IL-4, IL-5, and IL-10, unexpectedly. The colony-forming unit of the lung was decreased exponentially in both strains, and that of Brown Norway rats was significantly lower than that of Lewis rats 10 days after the inoculation. This indicated that Brown Norway rats demonstrated more fungicidal activity than Lewis rats in the early stage of the infection. The role of eosinophils with humoral immunity may be considered to be resistant in Brown Norway rats in addition of the function of macrophages.
Similar content being viewed by others
References
Yamaoka H, Sakaguchi N, Sano K, ItoM. Intravascular granuloma induced by intravenous inoculation of Cryptococcus neoformans. Mycopathologia 1996; 133: 149-158.
Huffnagle GB, Toews GB, Burdick MD, Boyd MB, McAllister KS, McDonald RA, Kunkel SL, Strieter RM. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol 1996; 157: 4529-4536.
Bulmer GS, Tacker JR. Phagocytosis of Cryptococcus neoformans by alveolar macrophages. Infect Immun 1975; 11: 73-79.
Kozel TR, Highison B, Stratton CJ. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun 1984; 43: 574-579.
Chen GH, Curtis JL, Mody CH, Christensen PJ, Armstrong LR, Toews GB. Effect of granulocyte-macrophage colonystimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J Immunol 1994; 152: 724-734.
Flesch IE, Schwamberger G, Kaufmann SH. Fungicidal activity of IFN-γ-activated macrophages: extracellular killing of Cryptococcus neoformans. J Immunol 1989; 142: 3219-3224.
Adams DO. The granulomatous inflammatory response: A review. Am J Pathol 1976; 84: 164-191.
Lohoff M, Gessner A, Bogdan C, Röllinghoff M. The Thl/Th2 paradigm and experimental murine leishmaniasis. Int Arch Allergy Immunol 1998; 115:191-202.
Chensue SW, Warmington KS, Ruth JH, Lincoln P, Kunkel SL. Cytokine function during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation: local and regional participation of IFN-γ, IL-10, and TNF. J Immunol 1995; 154: 5969-5976.
Chensue SW, Ruth JH, Warmington K, Lincoln P, Kunkel SL. In vivo regulation of macrophage IL-12 production during type 1 and type 2 cytokine-mediated granuloma formation. J Immunol 1995; 155: 3546-3551.
Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL. Mycobacterial and sch istosomal antigen-elicited granuloma 130 formation in IFN-γ and IL-4 knockout mice. J Immunol 1997; 159: 3565-3573.
Murphy JW, Bistoni F, Deepe GS, Blackstock RA, Buchanan K, Ashman RB, Romani L, Mencacci A, Cenci E, Fe d'Ostiani C, Del Sero G, Calich VL, Kashino SS. Type 1 and type 2 cytokines: from basic science to fungal infections. Med Mycol 1998; 36 (Suppl 1):109-118.
Damoiseaux JG, Beijleveld LJ, Schuurman HJ, van Breda Vriesman PJ. Effect of in vivo rapamycin treatment on de novo T-cell development in relation to induction of autoimmunelike immunopathology in the rat. Transplantation 1996; 62: 994-1001.
Saoudi A, Bernard I, Hoedemaekers A, Cautain B, Martinez K, Druet P, De Baets M, Guery JC. Experimental autoimmune myasthenia gravis may occur in the context of a polarized Th1-or Th2-type immune response in rats. J Immunol 1999; 162: 7189-7197.
Beijleveld LJ, Groen H, Broeren CP, Klatter FA, Kampinga J, Damoiseaux JG, van Breda Vriesman PJ. Susceptibility to clinically manifest cyclosporine A (CsA)-induced autoimmune disease is associated with interferon-gamma (IFN-γ )-producing CD45RC+RT6- T helper cells. Clin Exp Immunol 1996; 105: 486-496.
Hall E, Parton R, Wardlaw AC. Differences in coughing and other responses to intrabronchial infection with Bordetella pertussis among strains of rats. Infect Immun 1997; 65: 4711-4717.
Anderson JR. Muir's textbook of pathology, 12th edn. London: Edward Arnold (publishers) Ltd, 1985.
Mody CH, Tyler CL, Sitrin RG, Jackson C, Toews GB. Interferon-γ activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol 1991; 5: 19-26.
Decken K, Koöhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G. lnterleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun 1998; 66: 4994-5000.
Hoag KA, Street NE, Huffnagle GB, Lipscomb MF. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol 1995; 13: 487-495.
Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol 1997; 17: 733-739.
Ide K, Hayakawa H, Yagi T, Sato A, Koide Y, Yoshida A, Uchijima M, Suda T, Chiba K, Nakamura H. Decreased expression of Th2 type cytokine mRNA contributes to the lack of allergic bronchial inflammation in aged rats. J Immunol 1999; 163: 396-402.
Feldmesser M, Casadevall A, Kress Y, Spira G, Orlofsky A. Eosinophil-Cryptococcus neoformans interactions in vivo and in vitro. Infect Immun 1997; 65: 1899-1907.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kobayashi, M., Ito, M., Sano, K. et al. Granulomatous and cytokine responses to pulmonary Cryptococcus neoformans in two strains of rats. Mycopathologia 151, 121–130 (2001). https://doi.org/10.1023/A:1017900604050
Issue Date:
DOI: https://doi.org/10.1023/A:1017900604050