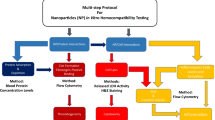
Abstract
A multi-parametric, multi-center evaluation of three polymers was performed measuring their response to blood contact. The purpose of this study was to pinpoint differences in tests performed for assessing “basic” hemocompatibility on identical materials at different centers and attempt to rationalize. Assays for platelet adhesion, activation, aggregability and activation of the coagulation system in addition to an ex vivo patency assay were performed at four centers across Europe, using protocols favored by each center for determining the blood-contacting performance of a biomaterial. Three polymers were chosen for their expected blood response spanning the range of undesirable to desirable: ethylenevinylacetate (EVA), polyvinylchloride (PVC) and PVC modified with polyethylene oxide (PEO). The assays were ranked in terms of their efficacy compared to cost and simplicity. A correlation between assays was calculated, indicating the ability of one test to correctly determine the blood response compared to another. Some assays were unable to distinguish between materials, but of the assays which could, the materials were ranked in the following order: EVA; PVC; PVC-PEO, EVA producing the most undesirable response. It is concluded that many commonly used assays for determining hemocompatibility are inappropriate, but there are simple and reliable test methods available which correlate well with the more sophisticated protocols.
Similar content being viewed by others
References
S. Dawids, “Test Procedures for the Blood Compatibility of Biomaterials”, edited by S. Dawids (Kluwer Academic Publishers, Dordrecht, 1993).
S. Dawids and A. Bantjes, “Blood Compatible Materials and their Testing”, edited by S. Dawids and A. Bantjes (Martinus Nijhoff Publishers, Dordrecht, 1986).
C. J. Kirkpatrick, Med. Dev. Tech. 1(5) (1998) 38.
D. F. Williams, “Techniques of biocompatibility testing”, edited by D. F. Williams (Boca Raton, Florida, CRC Press, 1986).
C. Baquey, B. Dupuy, G. Janvier, L. Bordenave, J. Caix and B. Basse-Cathalinat, Biorheology 28(5) (1991) 463.
J. Black, “Biological performance of materials: fundamentals of biocompatibility”, edited by J. Black (M. Dekker, New York, 1981).
D. F. Williams, “Biocompatibility of clinical implant materials”, edited by D. F. Williams (Boca Raton, Florida, CRC Press, 1981).
E. W. Salzman, Blood 38(4) (1971) 509.
Y. Nose, Artif. Organs 15(1) (1991) 1.
H. Klinkmann, Contrib Nephrol. (1984).
D. Hill, Mater. Dev. Tech. Jan.–Feb. (1995) 14.
J. M. Courtney, N. M. K. Lamba, S. Sundaram and C. D. Forbes, Biomaterials 15(10) (1994) 737.
J. M. Anderson, J. Lab. Clin. Med. 110(6) (1987) 666.
V. I. Sevastianov, E. A. Tseytina, A. V. Volkov and V. I. Shumakov, Trans. Am. Soc. Artif. Intern. Organs 30 (1984) 137.
J. L. Brash in “Modern Aspects of Protein Adsorption on Biomaterials” (Kluwer Academic Publishers, Dordrecht, 1991) p. 39.
Y. F. Missirlis and J. L. Wautier, “The Role of Platelets in Blood-Biomaterial Interactions”, edited by Y. F. Missirlis and J. L. Wautier (Kluwer Academic Publishers, Dordrecht, 1993).
J. A. Davies, in “Blood-Surface Interaction: Biological Principles Underlying Haemocompatibility with Artificial Materials” (Elsevier Science Publishers, Amsterdam, 1986).
P. Didisheim, M. K. Dewanjee, C. S. Frisk, M. P. Kaye and D. N. Fass, in “Contemporary Biomaterials” (Noyes Publications, Park Ridge, 1984) p. 132.
S. L. Cooper, D. J. Fabrizius and T. G. Grasel, in “Blood in contact with natural and artificial surfaces” (Ann. N.Y. Acad. Sciences, 516, 1987) p. 572.
M. K. Dewanjee, in “Blood in contact with natural and artificial surfaces” (Ann N.Y. Acad. Sciences, 516, 1987) p. 541.
H. L. Goldsmith and V. T. Turitto, Thromb. Haemost. 55 (1986) 415.
S. M. Slack and V. T. Turitto, Thromb. Haemost. 72(5) (1994) 777.
COMMITTEE DRAFT ISO 10993-4, 1999 Biological evaluation of medical devices-Part 4: Selection of tests for interactions with blood.
NIH Guidelines for blood/material interactions Publication No. 85-2185, 1985.
S. J. Northup, Intern. J. Toxicology 18(4) (1999) 275.
M. J. Buchanan, P. J. Upman and R. F. Wallin, Med. Dev. & Diagn. Ind. Mag. (Nov. 1998).
J. N. Mulvihill, A. Poot, T. Beugeling, A. Bantjes, W. G. Van Aken and J. P. Cazenave, in “Test Procedures for the Blood Compatibility of Biomaterials” (Kluwer Academic Publishers, Dordrecht, 1993) p. 377
N. P. Rhodes, T. V. Kumary and D. F. Williams, Biomaterials 17(20) (1996) 1995.
Y. F. Missirlis and G. P. A. Michanetzis, in “The Reference Materials of the European Communities” (Kluwer Academic Publishers, Dordrecht, 1992) p. 157.
G. P. A. Michanetzis and Y. F. Missirlis, J. Mater. Sci. Mater. in Med. 7(1) (1996) 29.
T. Schulze, W. Lemm and E. S. Bucherl, Life Support Syst. 1 (1983) 231.
W. Lemm, in “The Reference Materials of the European Communities” (Kluwer Academic Publishers, Dordrecht, 1992) p. 173.
M. C. Rissoan, R. Eloy and J. Baguet, in “Test Procedures for the Blood Compatibility of Biomaterials” (Kluwer Academic Publishers, Dordrecht, 1993) p. 461.
B. Dudley, J. L. Williams, K. Able and B. Muller, Trans. Amer. Soc. Artif. Int. Organs XXII (1976) 538.
K. S. Sakariassen, P. A. Bolhuis and J. J. Sixma, Thromb. Res. 19 (1980) 547.
K. L. Kaplan and J. Owen, Blood 57 (1981) 199.
R. P. McEver and M. N. Martin, J. Biol. Chem. 259 (1984) 9799.
J. P. Cazenave, J. N. Mulvihill, A. Sutter-Bay, C. Gachet, F. Tob and A. Beretz, in “Tests Procedures for the Blood Compatibility of Biomaterials” (Kluwer Academic Publishers, Dordrecht, 1993) p. 359.
M. J. Galloway, B. A. McVerry and M. J. Mackie, Thromb. Res. 33 (1984) 229.
N. P. Rhodes and D. F. Williams, Biomaterials 15(1) (1994) 34.
V. Turitto, H. J. Weiss and H. R. Baumgartner, J. Rheol. 23 (1979) 735.
V. Turitto and H. R. Baumgartner, Trans. Am. Soc. Artif. Intern. Org. 21 (1975) 593.
J. M. Courtney, M. Travers, J. T. Douglas, G. D. O. Lowe, C. D. Forbes, M. Aslam and C. J. Ryan, in “Blood Compatible Materials and their Testing” (Martinus Nijhoff Publishers, Dordrecht, 1986) p. 135.
K. D. Nelson, R. Eisenbaumer, M. Pomerantz and R. C. Eberhart, ASAIO J. 42(5) (1996) 884.
J. H. Lee, H. B. Lee and J. D. Andrade, Prog. in Polym. Sci. 20(6) (1995) 1043.
D. K. Han, K. D. Park, G. H. Ryu, U. Y. Kim, M. G. Min and Y. H. Kim, J. Biomed. Mater. Res. 30(1) (1996) 23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michanetzis, G.P.A., Missirlis, Y.F., Rhodes, N.P. et al. Influence of test protocol in determining the blood response to model polymers. Journal of Materials Science: Materials in Medicine 13, 757–765 (2002). https://doi.org/10.1023/A:1016166807299
Issue Date:
DOI: https://doi.org/10.1023/A:1016166807299