Abstract
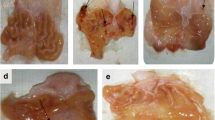
Five experimental models were developed in different groups of Wistar rats (N = 15) to study selective COX-2-inhibitor NSAIDs such as celecoxib and rofecoxib, as follows: (1) dose-dependent oral Celecoxib and Rofecoxib for 5 days, and 24 hr after oral indomethacin; (2) Same as 1 but subcutaneously; (3) gastric ulcer induced by glacial acetic acid; (4) duodenal ulcer induced by cysteamine; and (5) stress by immobilization and immersion in water at 15°C for 6 hr. Celecoxib and Rofecoxib, either orally or subcutaneously, did not produce necrotic lesions in healthy gastrointestinal mucosa (0%), showing normal histology. In contrast, previously indomethacin-induced lesions were aggravated (90%, P < 0.001). Total necrosis in the small intestine as well as increased ulcers and perforation of gastric and duodenal ulcers induced by acetic acid and cysteamine were observed. There was also aggravation of the necrotic gastric area in stress (60–90%, P < 0.05). Celecoxib and rofecoxib showed neutrophilia (5000/mm3) similar to that with indomethacin. In contrast, there was no leukocyte infiltration in the gastric mucosa; thus, we can consider it a selective COX-2 NSAID. In conclusion, celecoxib and rofecoxib at doses causing COX-2 but not COX-1 inhibition did not produce toxic lesions in healthy gastrointestinal mucosa, yielding a broad therapeutic margin. In contrast, when administered in altered gastrointestinal mucosa, they aggravated and complicated gastric ulcers as well as necrosis in the small intestine, consequently restricting their clinical use.
Similar content being viewed by others
REFERENCES
Fu JV, Masferrer JL, Seiberk K, Needleman P: The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem 265:16737–16740, 1990
Xie W, Chipman JG, Roberstson DL, Erikson RL, Simmons DL: Expression of a mitogen-responsive gene encording prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA. 88:1692–1696, 1991
Seibert K, Zhang Y, Leahy K, Hanser S, Masferrer JL, Perkins W, Lee L, Isakson P: Pharmacologial and biochemical demonstration of the role of cyclooxigenase 2 in inflammation and pain. Proc Natl Acad Sci USA 91:12013–12017, 1994
Hla T, Neilson K: Human cyclooxygenase 2 cDNA. Proc. Natl Acad Sci USA 266:7384–7388, 1992
Masferrer JL, Zweifel BS, Manning PT, Hanser SD, Leahy KM, Simith WS, Isakson PC, Serberk K: Selective inhibition of inducible cyclooxygenase 2 in vitro is anti-inflammatory and nonulcerogenic. Proc Natl Acad Sci USA 91:3228–3232, 1994
Meade EA, Smith WLL, Dewoitt DL: Differential inhibition of prostaglandin endoperoxidase syntehase (cyclooxygenase) isozymes by aspirin and other nonsteroidal anti-inflammatory drugs. J Biol Chem 95(suppl 2A):40–45, 1993
Michell JA, Akaraserremont P, Thiemerman C, Flower RJ, Vane JR: Selectivity of non-steroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA 90:11693–11697, 1993
Hawkey CJ: COX-2 inhibitors. Lancet 353:307–314, 1999
Singh G: NSAID-induced G1 complications: The ARAMIS perspective-1997. J Rheumatol 25(suppl 51):8–16, 1997
Bedini O, Laudanno OM, Naves P, San Miguel P, Cesolari J: Alteración de la angiogénesis y retardo en la reparación de la úlcera gástrica por úcido acético, en ratas tratadas con Meloxicam. Acta Gastroenteral Latino-Am 27(suppl 3):183A, 1997
Laudanno OM, Cesolari J, Esnarriaga JM, San Miguel P, Bedini O: Meloxicam (COX-2) vs indomethacin (COX-1) and gastroduodenal ulcer, in rats. Biocell 22(no 1):32A. 1998
Satoh H, Inada I, Hirata T, Maki Y: Indomethacin produces gastric antral ulcers in the refed rat. Gastroenterology 81:719–725, 1981
Laudanno OM, Cesolari JA, Esnarriaga J, Flaherty P, Vada J, Guastalli G, San Miguel P, Bedini O: Selectividad in vitro de DAINEs COX-1, COX-2 y úlceras gastrointestinales en ratas. Acta Gastroenterol Latino-Am 28:249–255, 1998
Okabe S, Roth, Pfeiffer CJ: A method for experimental, pen-etrating gastric and duodenal ulcers in rats. Am J Dig Dis. 16:277–284, 1971
Takagi K, Okabe S: The effect of drug on the production and recovery processes of stress ulcer. Jpn J Pharmacol 18:9–18, 1968
Krawisz JE, Sharon P, Stenson WF: Quantitative assay for acute inflammation based on myeloperoxidase activity: Assessment of inflammation in rats and hamster. Gastroenterology 87:1344–1350, 1984
Wallace JL, Bak A, Mcknight W, Asfaha S, Sharkey KA, MACNaughton WK: Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: Implications for gastrointestinal toxicity. Gastroenterology 115:101–109, 1998
Chan CC, Boyce S, Brideau C, et al: Rofecoxib: A potent and orally active cyclooxygenase-2inhibitor: Pharmacological and biochemical profiles. Pharmacol Exp Ther 290:551–560, 1999
Mc Adams BF: Systemic biosynthesis of prostaglandin by cyclooxygenase 2 (COX-2). The human pharmacology of a selective inhibition of COX-2. Proc Natl Acad Sci USA 96:272–277, 1999
Esnarriaga JM, Laudanno OM, Maglione C, Cesolari J, Guastalli G, Piombo G: Neutrophils in indomethacin gastric injury, in rats. Biocell 23(1):63A, 1999
Trevethick MA, Clayton NM, Strong P, Harman IW: Do infiltrating neutrophils to pathogenesis of indomethacin induce ulceration of the rat gastric antrum?. Gut 34:156–160, 1993
Laudanno OM, Cesolari JA, Esnarriaga J, San Miguel P, Bedini O: Gastric antrum and indomethacin ulcers, in rats. Digestion 80:1272A, 1998
Satoh H, Guth PH: Gastric antral ulcers produced by indomethacin in the rat. III. Role of acid and prostaglandin. Gastroenterology 80:1272A, 1981
Hayllar J, Byarmason I: NSAIDs, COX-2 inhibitors, and the gut. Lancet 346:521–522, 1995
Schreider HT, Nuernberg B, Dietzel K, Brune K: Biliary elimination of nonsteroidal anti-inflammatory drugs in patients. Br J Clin Pharmacol 29:1277–1281. 1990
Mizuno H, Sakamoto C, Matsuda K, Wada K, Hahida T, Noguahi H, Akamatsu T, Kasuga M: Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by specific antagonist delays healing in mice. Gastroenterology 112:387–397, 1997
Chang AD, Ramanujam KS, Wilson KT: Co-expression of inducible nitric oxide synthase (INOS), cyclooxygenase (COX-2), and TGF-B in rat models of colitis. Gastroenterology 110:881A, 1996 (abstract)
Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL: Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclo-oxygenase-2. J Clin Invest 98:2076–2085, 1996
Schmedtje JF Jr, Ji YS, Liu WL, DuBois RN, Runge MS: Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcriptor factor in human vascular endothelial cells. J Biol Chem 272:601–608, 1997
Fu S, Ramanujam KS, Wong A, et al: Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 116:1319–1329, 1999
Ramanujam KS, Wilson KT: Helicobacter pylori induces cyclooxygenase-2 expression in human gastric epithelial cells by a tyrosine phosphorylation-dependent mechanism. Gastroenterology 114:666A, 1998 (abstract)
Jackson LM, Wu K, Mahida YR, et al: COX-1 expression in human gastric mucosa infected with Helicobacter pylori: Constitutive or induced? Gastroenterology 114:160A, 1998
Plummer SM, Hall H, Faux SP: Oxidation and genotoxicity of fecapentaene-12 are potentiated by prostaglandin H synthase. Carcinogenesis 16:1023–1028, 1995
Boolbol SK, Dannenberg AJ, Chadburn A, et al: Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res 56:2556–2560, 1996
Ristimaki A, Honkanen H, Jaukala H, Sepponen P, Harkonen M: Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 57:1276–1280, 1997
Eberhart CE, Coffey RY, Radhica A, Giardello FM, Ferrenbach S, DuBois R N: Up regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107:1183–1188. 1994
Wilson KT, Fu S, Ramanujam KS, Meltzer SJ: Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 58:2929–2934, 1998