Abstract
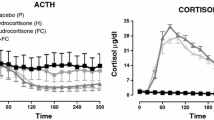
We have studied the in vivo influence of granulocyte-macrophage colony stimulating factor (GM-CSF) on blood plasma concentration of adrenocorticotropic hormone (ACTH) and corticosterone in Wistar rats. The administration of 10 µg/kg b.w. GM-CSF at 45 (P < 0.001), 90 (P < 0.01) and at 45 (P < 0.01), 90 (P < 0.001) and 180 min (P < 0.001) increased the secretion of ACTH and corticosterone, respectively. Prolonged administration of 10 µg/kg b.w. of GM-CSF increased the ACTH (P < 0.001) and corticosterone (P < 0.001) concentration in blood plasma. We have also found that chronic treatment with 10 µg/kg b.w. of GM-CSF increased the proliferative activity of corticotrophs (P < 0.05), but it did not significantly change the total cell proliferation in the anterior pituitary gland. Moreover, this cytokine increased cell proliferation of the adrenal cortex (P < 0.001). These experiments suggest that GM-CSF activates the pituitary-adrenal axis and support the hypothesis of bidirectional associations between the immune and neuroendocrine systems.
Similar content being viewed by others
References
Bateman A, Singh A, Krat T, Solomon S. The immune-hypothalamic-adrenal axis. Endocr Rev 1989;10:92–112.
Denicoff KD, Durkin TM, Lotze MT, Quinlan PE, Davis CL, Listwak SJ, Rosenberg SA, Rubinow DR. The neuroendocrine effects of interleukin-2 treatment. J Clin Endocrinol Metab 1989;69:402–410.
Gisslinger H, Svoboda T, Clodi M, Gilly B, Ludwig H, Havelec L, Luger A. Interferon-a stimulates the hypothalamicpituitary-adrenal axis in vivo and in vitro. Neuro Endocrinology 1993;57:489–495.
Gonzalez MC, Riedel M., Rettori V, Yu WH, McCann SM. Effect of recombinant human gamma interferon on the release of anterior pituitary hormones. PNEI 1990;3:49–54.
Holsboer F, Stalla GK, Von Bardeleben V, Hammann K, Miler H, Miller OA. Acute adrenocortical stimulation by recombinant gamma interferon in human controls. Life Sci 1988;42:1–5.
Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin releasing factor. Science 1987;238:522–524.
Sweep F, Rijnkels C, Hermus A. Activation of the hypothalamus-pituitary-adrenal axis by cytokines. Acta Endocrinol 1991;125:84–91.
Hall NR, Goldstein AL. Endocrine regulation of host immunity: The role of steroids and thymosin. In: Feniche RL, Chirigos AM (eds). Immune Modulation Agents and Their Mechanism. New York: Marcell Dekker 1984;533.
Devereux S, Linch DC. Clinical significance of the haemopoietic growth factors. Br J Cancer 1989;61:2–5.
Gasson JC. Molecular physiology of granulocyte-macrophage colony stimulating factor. Blood 1991;77:1131–1145.
Metcalf D. Control of granulocytes and macrophages: molecular, cellular and clinical aspects. Science 1991;254:529–533.
Hamilton JA. Colony stimulating factors and monocytemacrophages, cytokines-Some controversies. Immun Today 1993;14:18–24.
Hansen PB, Johnson HE, Ralfkiaer E, Hansen NE. Blood neutrophil increment after a single injection of rhG-CSF or rhGM-CSF correlates with marrow cellularity and may predict the grade of neutropenia after chemotherapy. Br J Haematol 1993;84:581–585.
Robak T, ed. Biologia i farmakologia cytokin. WarszawaŁódź: PWN, 1995(in Polish).
Żylińska K, Komorowski J, Robak T, Mucha S, Stępień H. Effect of granulocyte-macrophage colony stimulating factor and granulocyte colony stimulating factor on melatonin secretion in rats. In vivo and in vitro studies. J Neuroimmunol 1995;56:187–190.
Bravo R, Frank R, Blundell PA, McDonald-Bravo H. Cyclin/ PCNA is the auxiliary protein of DNA polymerase δ. Nature 1987;326:515–517.
Taniguchi Y, Tamatani R, Yasutaca S, Kawarai Y. Proliferation of pituitary corticotrophs following adrenalectomy as revealed by immunohistochemistry combined with bromodeoxyuridine labeling. Histochemistry 1995;103:127–130.
Oishi Y, Okuda M, Takahashi H, Fujii T, Mori S. Cellular proliferation in the anterior pituitary gland of normal adult rats: influences of sex, estrous cycle, and circadian change. Anat Record 1993;235:111–120.
Sweep CGJ(F), Van der Meer MJM, Hermus ARMM, Smals AGH, Van der Meer JWM, Pesman GJ, Willemsen SJ, Benraad TJ, Kloppenborg PWC. Chronic stimulation of the pituitary-adrenal axis in rats by interleukin-1b infusion: in vivo and in vitro studies. Endocrinology 1992;130:1153–1164.
Naito Y, Fukata J, Nakaishi S, Nakai Y, Hirai Y, Tamai S, Mori K, Imura H. Chronic effects of interleukin-1 on hypothalamus, pituitary and adrenal glands in rat. Neuro Endocrinology 1990;51:637–641.
Bernardini R, Kamilaris TC, Calogero AE, Johnson EO, Gold PW, Chrousos GP. Interaction between tumor necrosis factor-alpha, hypothalamic corticotropin releasing hormone and adrenocorticotropin secretion in the rat. Endocrinology 1990;126:2876–2881.
Imura H, Fukata J, Mori T. Cytokines and endocrine function: An interaction between the immune and neuroendocrine systems. Clin Endocrinol 1991;35:107–115.
Hoekman K, von Blomberg-van der Flier BME, Wagstaff J, Drexhage HA, Pinedo HM. Reversible thyroid dysfunction during treatment with GM-CSF. Lancet 1992;338:541–542.
Crispino S, Lissoni P, Ardizzoia A, Barni S, Rovelli F, Tancini G. Effects of granulocyte-macrophage colony-stimulating factor on cortisol, growth hormone, prolactin and melatonin in cancer patients. J Biol Regul Homeost Agents 1992;6:142–144.
Wautier JL, Vilette D, Caen JP. Modulation of endothelial cell proliferation by monocyte-derived cytokines. In: Simionescu N, Simionescu M (eds). Endothelial Cell Dysfunctions. New York: Plenum Press, 1992;169–181.
Saxena SK, Crouse DA, Sharp JG. Effect of systemic interleukin-3 administration on epithelial cell proliferation in mouse intestine. Life Sci 1993;53:473–477.
Zieleniewski W, Zieleniewski J, Stępień H. Effect of interleukin-1a, IL-1b and IL-1 receptor antibody on the proliferation and steroidogenesis of regenerating rat adrenal cortex. Exp Clin Endocrinol 1995;103:373–377.
Suda T, Tozawa F, Ushiyama T, Sumitomo T, Yamada M, Demura H. Interleukin-1 stimulates corticotropin-releasing factor gene expression in rat hypothalamus. Endocrinology 1990;126:1223–1228.
Arzt E, Buric R, Stelzer G, Stalla J, Sauer J, Renner U, Stalla GK. Interleukin involvement in anterior pituitary cell growth regulation: effects of IL-2 and IL-6. Endocrinology 1993;132:459–467.
StępieńH, Żerek-Mełń G, Mucha S, Winczyk K, Fryczak J. Interleukin-1b stimulates cell proliferation in the intermediate lobe of the rat pituitary gland. J Endocrinol 1994;140: 337–341.
Kunert-Radek J, Radek A, Stępień H. Interluekin-2 stimulates cell proliferation of the growth hormone producing human pituitary adenoma in vitro. Biomed Lett 1994;49:259–264.
Dedhar S, Gaboury L, Galloway P., Eaves C. Human granulocyte-macrophage colony stimulating factor is active on a variety of all types of nonhematopoietic cells. Proc Natl Acad Sci USA 1988;85:9253–9257.
Berdel WE, Danhauser-Riedl S, Steinhauser G, Winton EF. Various human hematopoietic growth factors (IL-3, GMCSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood 1989;73:80–83.
Guillemin G, Boussin FD, Le Grand R, Croitoru J, Coffigny H, Dormont D. Granulocyte macrophage colony stimulating factor stimulates in vitro proliferation of astrocytes derived from simian mature brains. Glia 1996;16:71–80.
Hunt TE. Mitotic activity in the anterior hypophysis of female rats of different age groups and at different periods of the day. Endocrinology 1943;32:334–339.
Childs GV, Rougeau D, Unabia G. Corticotropin-releasing factor and epidermal growth factor: mitogens for anterior pituitary corticotropes. Endocrinology 1995;136:1595–1602.
Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today 1994;15:504–511.
Imura H, Fukata J. Endocrine-paracrine interaction in communication between the immune and endocrine systems. Activation of the hypothalamic-pituitary-adrenal axis in inflammation. Eur J Endocrinol 1994;130:32–37.
Besedovsky HO, del Rey AE, Sorkin E. Immune-neuroendocrine interactions. J Immunol 1985;135:750–754.
Reichlin S. Neuroendocrine-immune interactions. N Engl J Med 1993;329:1246–1253.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Żylińska, K., Mucha, S., Komorowski, J. et al. Influence of Granulocyte-Macrophage Colony Stimulating Factor on Pituitary-Adrenal Axis (PAA) in Rats In Vivo.. Pituitary 2, 211–216 (1999). https://doi.org/10.1023/A:1009905427902
Issue Date:
DOI: https://doi.org/10.1023/A:1009905427902