Abstract
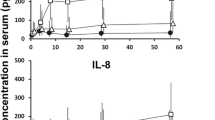
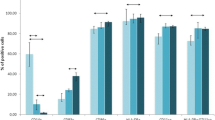
We investigated possible mechanisms leading to the inhibition of theimmune system in people with chronic disorders. Tumor cell produce proteinreleased into the circulation, such as tumor associated antigens, may playan important role in processes preceding paralysis of the immune system. Totest this hypothesis the following tumor associated antigens were used: AFP,OFP, CA-125, CA-50 and CA-19-9. Their role was assessed by modulatingcytokine production in cord blood lymphocytes and peripheral white bloodcells obtained from grown population of patients treated with colostrinin,an cytokine inducer. PHA, LPS and colostrinin were used as positive controlin those essays. Each antigen tested individually induced IFN, TNFαand IL-6 in dose dependent fashion. None of the tested cytokines werespontaneously released by the cells. Data generated from these experimentsindicated that tumor associated antigens are inducing type 1 cytokines insimilar fashion as LPS or colostrinin. However, lymphocytes taken frompatients undergoing therapy with colostrinin revealed progressive losscapability to produce type 1 cytokines as they did in case of colostrinin.The loss of the capability to respond to antigen may represent phenomenonleading to immune tolerance.
Similar content being viewed by others
References
Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer JP. Colorectal carcinoma antigens detected by hybridoma antibodies. Somat. Cell. Mol. Gen. 1979; 5: 957–972.
Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science 1981; 212: 53–55.
Atkinson BF, Ernst CS, Herlyn M, Steplewski Z, Sears HF, Koprowski H. Gastrointestinal cancer-associated antigen in immunoperoxidase assay. Cancer Res 1982; 42: 4820–4823.
Magnani JL, Steplewski Z, Koprowski H, Ginsburg V. Identifi-cation of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983; 43: 5489–5492.
Olding LB, Thurin J, Svalander C, Koprowski H. Expression of gastrointestinal carcinoma-associated antigen (GICA) detected in human fetal tissues by monoclonal antibody. NS-19-9. Int. J. Cancer 1984; 34: 187–192.
Raux H, Labbe F, Fondaneche MC, Koprowski H, Burtin P. A study of gastrointestinal cancer-associated antigen (GICA) in human fetal organs. Int. Cancer 1983; 32: 315–319.
Tizzani A, Casetta G, Ciciogoi A, Piana P, Cerchier A, Pecchio F, Piantino P. Tumor markers (CEA, TPA and CA 19-9) in urine of bladder cancer patients. Int. J. Biol. Markers 1987; 2: 121–124.
Hakomori S. Possible functions of tumor-associated carbohydrate antigens. Curr. Opin. Immunol 1991; 3: 646–653.
Nakayama T, Watanabe M, Katsumata T, Teramoto T, Kitajima M. Expression of sialyl Lewis(a) as a new prognostic factor for patients with advanced colorectal carcinoma. Cancer 1995; 75: 2051–2056.
Klopocki AG, Krop-Watorek A, Dus D, Ugorski M. Adhesion of human uroepithelial cells to E-selectin: possible involvment of sialosyl Lewis a-ganglioside. Int. J. Cancer 1996; 68}: 239–
Janusz M, Staroscik K, Zimecki M, Wieczorek Z, Lisowski J. Chemical and physical characterization of a proline-rich polypeptide from sheep colostrum. Biochem. J. 1981; 199: 9–15.
Janusz M, Lisowski J. Proline-rich polypeptide (PRP)-an immunomodulatory peptide from ovine colostrum. Arch. Immunol. et Ther. Expl. 1993; 41: 275–279.
Inglot AD, Janusz M, Lisowski J. Colostrinine: a proline-rich polypeptide from ovine colostrum is a modest cytokine inducer in human leukocytes. Arch. Immunol. et Ther. Expl. 1996; 44: 215–224.
Piasecki E, Inglot AD, Winiarska M, Krukowska K, Janusz M, Lisowski J. Coincidence between spontaneous release of interferon and tumor necrosis factor by colostral leukocytes and the production ofa colostrinin by human mammary gland after normal delivery. Arch. Immunol. et Ther. Expl. 1997; 45: 109–117.
Georgiades JA, Gelder F, Inglot A. Isolation and preliminary characterization of a new cytokine in human colostrum; its similarity to ovine colostrinine. Eur. Cytokine Netw. 1996: 7: 511.
Stringfellow DA. Regulation of interferon production and hyporesponsiveness. Interferon System. Texas Reports on Biology and Med. Baron S, Dianzani N, Stanton GJ eds. Univ. of Texas, Austin, USA. 1997, 83–88.
Ho M, Kono Y, Breining MK. Tolerance to the induction of interferons by endotoxin and virus: role of a humoral factor. Proc. Soc. Exp. Biol. Med. 1965; 119: 1227–1232.
Levy HB, Salazar AM. Interferon inducers. Interferon: Principles and Medical Applications eds. Baron S. et al. (The University of Texas Medical Branch at Galveston Dept. of Microbiol. Galveston TX USA) 1992; 65–76.
Yamamoto JK, Kruzel L, Louie H, Georgiades JA. Inhibition of human immunodeficiency virus type I replication by human interferons alpha, beta and gamma. Arch Immunol. et Ther. Expl 1993; 41: 185–192.
Leszek J, Inglot AD, Janusz M, Lisowski J. Therpeutic effi-cacy of colostrinine in Alzheimer's disease Suppl. to Biol. Psychiatry 1997, 42 (1S) Abstract 39–29.
Tishon A, Borrow P, Oldstone MBA. Virus-induced immuno-suppression. Age at infection relates to a selective or generalized defect. Virology 1993; 195: 397–405.
Cheynier R, Langlade-Demoyen P, Marescot MR, Blanche S, Blandin G, Wain-Hobson S, Griscelli C, Vilmer E, Plato F. Cytoxic T lymphocyte responses in the peripheral blood of children born to human immunodefciency virus-1-infected mothers. Europ. J. Immunol. 1992; 22: 2211–2217.
Field C, Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J. Hyg. 1978; 81: 267–277.
Penna A, Chisari FV, Bartoletti A, Missale G, Fowler O, Giuberti T, Ficcadori F, Ferrari C. Cytoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatiits B virus nucleocapsid antigen. J. Expl. Med. 1991; 174: 1565–1570.
Taylor PM, Davey J, Howland K, Ruthbard J, Ascona BA. Class I MHC molecules rather than other mouse genes dictate influeza epitope recognition by cytoxic T cells. Immunotherapies 1987; 26: 267–272.
Wu-Hsein B, Howard DH, Ahmed R. Virus-induced immunosupression a murine model of susceptibility to opportunistic infection. J. Infect Dis. 1988; 158: 232–235.
Teichman JV, Sieber G, Ludwig WD, Ruehl H. Immunosupressive effects of recombinant interferon-alpha during long-term treatment of cancer patients. Cancer 1989; 63: 1990–1993.
Gangopadhyay A, Bajenova O, Kelly TM, Thomas P. Carcinoembryonic antigen induces cytokine expression in Kuppfer cells: implications for hepatic metastasis from colorectal cancer. Cancer Res. 1996; 56: 4805–4810.
Bocci V. x of interferon? Clin. Pharmacokin. et. 1991; 21: (6) 411–417.
Goldman AB, Chheda S, Garafolo R, Schmalsteig FC. Cytokine in human milk: properties and potential effects upon the mammary gland and the neonate. J. Mamm. Gland Biol. and Neoplasia 1996; 1: 251–258.
Bocci V, Von Bremen K, Corradeschi E, Luzzi E, Paulescu L. What is the orle of cytokines in human colostrum? J. Biol. Regulators and Homeost Agents 1991; 5: (4) 121–124.
Garofolo R, Chheda S, Mei F, Palkowetz KH, Rudloff HE, Schmalsteig FC, Rassin DK Goldman AS. Interleukin-10 in human milk. Pediatric Res. 1995; 37: (4) 444–448.
Cembrzynska-Nowak M, Szklarz E, Inglot AD. Modulation of cytokine production by a selenoorganic compound (AE22) in hyperreactive or hyporeactive bronchio alveolar leukocytes of asthmatics or lung cancer patients J. Interferon Cyt. Res. 1997; 17: 609–617.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Inglot, A.D., Gelder, F. & Georgiades, J.A. Tumor-associated antigens are cytokine inducers and hyporeactivity factors to the immune system. Biotherapy 11, 27–37 (1998). https://doi.org/10.1023/A:1007936706416
Issue Date:
DOI: https://doi.org/10.1023/A:1007936706416