Abstract
Purpose. The purpose of this study was to demonstrate the potential of a dynamic, multicompartmental in vitro system simulating the human stomach and small intestine (TIM-1) for studying the behavior of oral drug dosage forms under various physiological gastrointestinal conditions.
Methods. Two model drug compounds were studied in TIM-1: a lyophilized Lactobacillus strain and paracetamol (acetaminophen). The Lactobacillus survival rate was determined by bacterial counting in the gastric and ileal effluents while simulating the conditions of the gastrointestinal tract of infants or adults. The availability for absorption of paracetamol from two oral dosage forms was investigated by measuring the drug concentration in jejunal dialysis fluid. The effect of gastrointestinal passage time and food intake on paracetamol absorption was also studied.
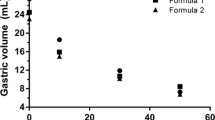
Results. The Lactobacillus survival rate in both gastric and ileal effluents was higher during simulation of the infant compared to adult conditions. We also showed that (i) paracetamol absorption was faster when it was administered as a free powder than in sustained-release tablet form, (ii) a slow passage time resulted in a delay in the absorption of paracetamol, and (iii) there was a lower rate of absorption when paracetamol was ingested with a standard breakfast as opposed to water. The in vitro results were consistent with in vivo data, showing the predictive value of TIM-1.
Conclusions. TIM-1 is a powerful tool for supplying valuable information about the effects of various gastrointestinal conditions on biopharmaceutical behavior and efficacy of drug delivery systems in the development of oral formulations.
Similar content being viewed by others
References
M. Minekus, P. Marteau, R. Havenaar, and J. H. J. Huis in't Veld. A multicompartmental dynamic computer-controlled modelsimulating the stomach and small intestine. Atla 23:197-209 (1995).
R. Havenaar and M. Minekus. In vitro model of an in vivo digestive tract. JP, US, European Patent PCT/NL93/00225 (1994).
L. Babinsky, J. M. Van Der Meer, H. Boer, and L. A. Den Hartog. An in vitro method for prediction of digestible crude protein content in pig feeds. J. Sci. Food Agric. 50:173-178 (1990).
H. N. Englyst, S. M. Kingman, G. J. Hudson, and J. H. Cummings. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 75:749-755 (1996).
J. Vatier, A. Harman, N. Castela, M. T. Droy-Lefaix, and R. Farinotti. Interactions of cimetidine and ranitidine with aluminium-containing antiacids and a clay-containing gastric-protective drug in an “artificial stomach-duodenum” model. J. Pharm. Sci. 83:962-966 (1994).
M. Yvon, S. Beucher, P. Scanff, S. Thirouin, and J. P. Pelisssier. In vitro simulation of gastric digestion of milk proteins: comparison between in vitro and in vivodata. J. Agric. Food Chem. 40:239-244 (1992).
K. Molly, M. Van de Woestyne, and W. Verstraete. Development of a 5-step multichamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 39:254-258 (1993).
A. C. Longland. Digestive enzymes in pigs and poultry. In M. F. Fuller (ed.), In Vitro Digestion for Pigs and Poultry, CAB International, Wallingford, UK, 1991, pp. 3-18.
M. J. E. Smeets-Peeters, M. Minekus, R. Havenaar, G. Schaafsma, and M. W. A. Verstegen. Description of a dynamic in vitro model of the dog gastrointestinal tract and an evaluation of various transit times for protein and calcium. Atla 27:935-949 (1999).
M. Larsson, M. Minekus, and R. Havenaar. Estimation of the bioavailability of iron and phosphorus in cereals using a dynamic in vitro gastrointestinal model. J. Sci. Food Agric. 74:99-106 (1997).
M. Verwei, K. Arkbage, R. Havenaar, H. Van den Berg, C. Witthöft, and G. Schaafsma. Folic acid and 5-methyl-tetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. J. Nutr. 133:2377-2383 (2003).
C. A. M. Krul, A. Luiten-Schuite, R. Baan, H. Verhagen, G. Mohn V. Feron, and R. Havenaar. Application of a dynamic in vitro gastrointestinal tract model to study the availability of food mutagens, using heterocyclic aromatic amines as model compounds. Food Chem. Toxicol. 38:783-792 (2000).
P. Marteau, M. Minekus, R. Havenaar, and J. H. J. Huis in't Veld. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of the bile. J. Dairy Sci. 80:1031-1037 (1997).
S. Blanquet, S. Marol-Bonnin, E. Beyssac, D. Pompon, M. Renaud, and M. Alric. The biodrug concept: an innovative approach to therapy. Trends Biotechnol. 19:393-400 (2001).
S. Blanquet, J. P. Meunier, M. Minekus, S. Marol-Bonnin, and M. Alric. Recombinant Saccharomyces cerevisiae expressing a P450in artificial digestive systems: a model for biodetoxication in the human digestive environment. Appl. Env. Microbiol. 69:2884-2892 (2003).
Nederlandse organisatie voor toegepast (TNO). Composition for controlled release substance and method for preparation of such a composition. European Patent 0 648 116 B1 (1996).
J. D. Elashoff, T. J. Reedy, and J. M. Meyer. Analysis of gastric emptying data. Gastroenterology. 83:1306-1312 (1982).
W. G. M. Boeijen. Nevo table. Nederlandse voedingstoffen bestand, TNO Voeding, Zeist, 1987.
M. P. Vaquero, W. Van Dokkum, K. D. Bos, M. G. E. Wolters, G. Schaafsma, and J. B. Luten. In vitro availability of calcium, magnesium, iron, copper and zinc from white or brown bread separately or in combination with other foods. Revista Espanola de Ciencia y Technologia de Alimentos. 32:47-58 (1992).
B. Goldin, S. Gorbach, M. Saxelin, S. Barakat, L. Gualtieri, and S. Salminen. Survival of Lactobacillus species (strain GG) inhuman gastrointestinal tract. Dig. Dis. Sci. 37:121-128 (1992).
P. Conway, S. Gorbach, and B. Goldin. Survival of lactic acidbacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 70:1-12 (1987).
P. Marteau, P. Pochard, Y. Bouhnik, S. Zidi, I. Goderel, and J. C. Rambaud. Survie dans l'intestin grêle de Lactobacillus acidophilus et de Bifidobacterium spp. ingérés dans un lait fermenté: une base rationnelle pour l'utilisation des probiotiques chez l'homme. Gastroenterol. Clin. Biol. 16:25-28 (1992).
T. Vesa, P. Pochart, and P. Marteau. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment Pharmacol. Ther. 14:823-828 (2000).
B. Ameer, M. Divoll, D. R. Abernethy, D. J. Greenblatt, and L. Shargel. Absolute and relative bioavailability of oral acetaminophen preparations. J. Pharm. Sci. 72:955-958 (1983).
A. Rostami-Hodjegan, M. R. Shiran, R. Ayesh, T. J. Grattan, I. Burnett, A. Darby-Dowman, and G. T. Tucker. A new rapidly absorbed paracetamol tablet containing sodium bicarbonate. I A four way crossover study to compare the concentration-time profile of paracetamol from the new paracetamol/sodium bicarbonate tablet and a conventional tablet in fed and fasted volunteers. Drug Dev. Ind. Pharm. 28:523-531 (2002).
T. Rygnestad, K. Zahlsen, and F. A. Samdal. Absorption of effervescent paracetamol tablets relative to ordinary paracetamol tablets in healthy volunteers. Eur. J. Clin. Pharmacol. 56:141-143 (2000).
E. M. Van Bommel, M. Raghoebar, and J. J. Tukker. Kinetics of acetaminophen after single-and multiple-dose oral administration as a gradient matrix system to healthy male subjects. Biopharm. Drug Dispos. 12:355-366 (1991).
M. Divoll, D. J. Greenblatt, B. Ameer, and D. R. Abernethy. Effect of food on acetaminophen absorption in young and elderly subjects. J. Clin. Pharmacol. 22:571-576 (1982).
J. M. Jaffe, J. L. Collaizzi, and H. Barry3rd. Effects of dietary components on GI absorption of acetaminophen tablets in man. J. Pharm. Sci. 60:1646-1650 (1971).
M. Minekus, M. Smeets-Peter, A. Bernalier, S. Marol-Bonnin, R. Havenaar, P. Marteau, M. Alric, G. Fonty, and J. H. J. Huis in't Veld. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl. Microbiol. Biotechnol. 53:108-114 (1999).
K. Venema, M. Van Nuenen, M. Smeets-Peeters, M. Minekus, and R. Havenaar. TNO's in vitro large intestinal model: an excellent screening tool for functional food and pharmaceutical research. Ernahrung/Nutrition 24:558-564 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blanquet, S., Zeijdner, E., Beyssac, E. et al. A Dynamic Artificial Gastrointestinal System for Studying the Behavior of Orally Administered Drug Dosage Forms Under Various Physiological Conditions. Pharm Res 21, 585–591 (2004). https://doi.org/10.1023/B:PHAM.0000022404.70478.4b
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000022404.70478.4b