Abstract
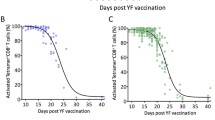
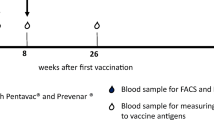
We investigated the humoral (antigen-specific immunoglobulin isotypes, IgG subclasses, and avidity maturation) and cellular (antigen-specific in vitro proliferation) immune response in 18 healthy adult volunteers, following a primary and a single booster vaccination with the T-cell dependent neoantigen rabies administered at a 3-months interval. The IgG antibody titer showed a mean 31-fold increase (range 3–154) 4 weeks after the first vaccination and a memory response was observed after booster vaccination, i.e. high IgG titers, switch from IgM to IgG and IgA and increased antibody avidity. All healthy adults showed a rabies-induced proliferative response with a mean stimulation index of 45 (range 3.5–200) after in vitro stimulation of PBMC obtained at 4 weeks after booster vaccination. The results obtained in this study provide a frame of reference for the interpretation of specific immune responses to the T-cell dependent neoantigen rabies in patients suspected of a primary or secondary immunodeficiency. Humoral and cellular immune responses to the rabies neoantigen provide complementary information on the condition of the immune system of an individual. Five patients diagnosed with a combined immunodeficiency were vaccinated using the same protocol and showed a number of abnormalities, either in the humoral or the cellular immune response to the rabies neoantigen.
Similar content being viewed by others
References
International Union of Immunological Societies. Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Clin Exp Immunol 118 Suppl 1:1–28, 1999
Uytdehaag FG, Osterhaus AD, Loggen HG, Bakker RH, van Asten JA, Kreeftenberg JG, van der MP, van Steenis B: Induction of antigen-specific antibody response in human peripheral blood lymphocytes in vitro by a dog kidney cell vaccine against rabies virus (DKCV). J Immunol 131:1234–1239, 1983
Perrin P, Joffret ML, Zanetti C, Bourhy H, Gontier C, Fritzell C, Leclerc C, Sureau P: Rabies-specific production of interleukin-2 by peripheral blood lymphocytes from human rabies vaccinees. Vaccine 9:549–558, 1991
Pyun KH, Ochs HD, Wedgwood RJ, Yang XQ, Heller SR, Reimer CB: Human antibody responses to bacteriophage phi X 174; Sequential induction of IgM and IgG subclass antibody. Clin Immunol Immunopathol 51:252–263, 1989
Weits J, de Gast GC, The TH, Esselink MT, Deelder AM, Petrovic M, Mandema E: Class-specific antibody titres (ELISA) against the primary immunogen Helix pomatia haemocyanin (HPH) in man. Clin Exp Immunol 32:443–450, 1978
Korver K, Boeschoten EW, Krediet RT, van Steenis G, Schellekens PT: Dose-response effects in immunizations with keyhole limpet haemocyanin and rabies vaccine: Shift in some immunodeficiency states. Clin Exp Immunol 70:328–335, 1987
Briggs DJ, Dreesen DW, Morgan P, Chin JE, Seedle CD, Cryz L, Gluck R, Cryz SJ: Safety and immunogenicity of Lyssavac Berna human diploid cell rabies vaccine in healthy adults. Vaccine 14:1361–1365, 1996
Plotkin SA: Rabies. Clin Infect Dis 30:4–12, 2000
Thisyakorn U, Pancharoen C, Wilde H: Immunologic and virologic evaluation of HIV-1-infected children after rabies vaccination. Vaccine 19:1534–1537, 2001
Chutivongse S, Wilde H, Benjavongkulchai M, Chomchey P, Punthawong S: Postexposure rabies vaccination during pregnancy: Effect on 202 women and their infants. Clin Infect Dis 20:818–820, 1995
Jones RL, Froeschle JE, Atmar RL, Matthews JS, Sanders R, Pardalos J, Moeller L, Chin JE, Famula M, Briggs DJ, Lang J: Immunogenicity, safety and lot consistency in adults of a chromatographically purified Vero-cell rabies vaccine: A randomized, double-blind trial with human diploid cell rabies vaccine. Vaccine 19:4635–4643, 2001
Sabchareon A, Lang J, Attanath P, Sirivichayakul C, Pengsaa K, Le MV, Chantavanich P, Prarinyanuphab V, Pojjaroen-Anant C, Nimnual S, Wood SC, Riffard P: A new Vero cell rabies vaccine: Results of a comparative trial with human diploid cell rabies vaccine in children. Clin Infect Dis 29:141–149, 1999
WHO Expert Committee on Rabies: World Health Organ Tech Rep Ser 824:1–84, 1992
Cejka J, Mood DW, Kim CS: Immunoglobulin concentrations in sera of normal children: Quantitation against an international reference preparation. Clin Chem 20:656–659, 1974
van Wezel AL, van Steenis G, Hannik CA, Cohen H: New approach to the production of concentrated and purified inactivated polio and rabies tissue culture vaccines. Dev Biol Stand 41:159–168, 1978
Lyng J, Bentzon MW, Ferguson M, Fitzgerald EA: Rabies vaccine standardization: International collaborative study for the characterization of the fifth International Standard for Rabies Vaccine. Biologicals 20:301–313, 1992
Jefferis R, Reimer CB, Skvaril F, de Lange GG, Goodall DM, Bentley TL, Phillips DJ, Vlug A, Harada S, Radl J,Claassen E, Boersma JA, Coolen J: Evaluation of monoclonal antibodies having specificity for human IgG subclasses: Results of the 2nd IUIS/WHO collaborative study. Immunol Lett 31:143–168, 1992
Lyng J: Calibration of a replacement preparation for the International Standard for Rabies Immunoglobulin. Biologicals 22:249–255, 1994
Pullen GR, Fitzgerald MG, Hosking CS: Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods 86:83–87, 1986
Rath S, Stanley CM, Steward MW: An inhibition enzyme immunoassay for estimating relative antibody affinity and affinity heterogeneity. J Immunol Methods 106:245–249, 1988
Luxton RW, Thompson EJ: Affinity distributions of antigen-specific IgG in patients with multiple sclerosis and in patients with viral encephalitis. J Immunol Methods 131:277–282, 1990
Korver K, Zeijlemaker WP, Schellekens PT, Vossen JM: Measurement of primary in vivo IgM-and IgG-antibody response to KLH in humans: implications of pre-immune IgM binding in antigen-specific ELISA. J Immunol Methods 74:241–251, 1984
Sagara J, Kawai A: Identification of heat shock protein 70 in the rabies virion. Virology 190:845–848, 1992
Bracci L, Ballas SK, Spreafico A, Neri P: Molecular mimicry between the rabies virus glycoprotein and human immunodeficiency virus-1 GP120; Cross-reacting antibodies induced by rabies vaccination. Blood 90:3623–3628, 1997
Martins TB, Jaskowski TD, Mouritsen CL, Hill HR: An evaluation of the effectiveness of three immunoglobulin G (IgG) removal procedures for routine IgM serological testing. Clin Diagn Lab Immunol 2:98–103, 1995
Bird P, Calvert JE, Amlot PL: Distinctive development of IgG4 subclass antibodies in the primary and secondary responses to keyhole limpet haemocyanin in man. Immunology 69:355–360, 1990
Ikematsu W, Kobarg J, Ikematsu H, Ichiyoshi Y, Casali P: Clonal analysis of a human antibody response. III. Nucleotide sequences of monoclonal IgM, IgG, and IgA to rabies virus reveal restricted V kappa gene utilization, junctional V kappa J kappa and V lambda J lambda diversity, and somatic hypermutation. J Immunol 161:2895–2905, 1998
Smith JS, Yager PA, Baer GM: A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ 48:535–541, 1973
Thraenhart O, Kreuzfelder E, Hillebrandt M, Marcus I, Ramakrishnan K, Fu ZF, Dietzschold B: Long-term humoral and cellular immunity after vaccination with cell culture rabies vaccines in man. Clin Immunol Immunopathol 71:287–292, 1994
Strady A, Lang J, Lienard M, Blondeau C, Jaussaud R, Plotkin SA: Antibody persistence following preexposure regimens of cell-culture rabies vaccines: 10-year follow-up and proposal for a new booster policy. J Infect Dis 177:1290–1295, 1998
Atanasiu P: Quantitative assay and potency test of antirabies serum and immunoglobulin. Monogr Ser World Health Organ 314–318, 1973
Briggs DJ, Schwenke JR: Longevity of rabies antibody titre in recipients of human diploid cell rabies vaccine. Vaccine 10:125–129, 1992
Rodrigues FM, Mandke VB, Roumiantzeff M, Rao CV, Mehta JM, Pavri KM, Poonawalla C: Persistence of rabies antibody 5 years after preexposure prophylaxis with human diploid cell antirabies vaccine and antibody response to a single booster dose. Epidemiol Infect 99:91–95, 1987
Aoki FY, Rubin ME, Fast MV: Rabies neutralizing antibody in serum of children compared to adults following postexposure prophylaxis. Biologicals 20:283–287, 1992
Lang J, Duong GH, Nguyen VG, Le TT, Nguyen CV, Kesmedjian V, Plotkin SA: Randomised feasibility trial of preexposure rabies vaccination with DTP-IPV in infants. Lancet 349:1663–1665, 1997
Ratanavongsiri J, Sriwanthana B, Ubol S, Phanuphak P: Cell-mediated immune response following intracutaneous immunisation with human diploid cell rabies vaccine. Asian Pac J Allergy Immunol 3:187–190, 1985
Phanuphak P, Khawplod P, Sirivichayakul S, Siriprasomsub W, Ubol S, Thaweepathomwat M: Humoral and cell-mediated immune responses to various economical regimens of purified Vero cell rabies vaccine. Asian Pac J Allergy Immunol 5:33–37, 1987
Nicholson KG, Cole PJ, Turner GS, Harrison P: Immune responses of humans to a human diploid cell strain of rabies virus vaccine: Lymphocyte transformation, production of virus-neutralizing antibody, and induction of interferon. J Infect Dis 140:176–182, 1979
Ghaffari G, Passalacqua DJ, Bender BS, Briggs DJ, Goodenow MM, Sleasman JW: Human lymphocyte proliferation responses following primary immunization with rabies vaccine as neoantigen. Clin Diagn Lab Immunol 8:880–883, 2001
Lafon M, Lafage M, Martinez-Arends A, Ramirez R, Vuillier F, Charron D, Lotteau V, Scott-Algara D: Evidence for a viral superantigen in humans. Nature 358:507–510, 1992
Cunningham-Rundles C, Bodian C: Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 92:34–48, 1999
Sneller MC: Common variable immunodeficiency. Am J Med Sci 321:42–48, 2001
Ochs HD, Nonoyama S, Farrington ML, Fischer SH, Aruffo A: The role of adhesion molecules in the regulation of antibody responses. Semin Hematol 30:72–79, 1993
Claassen IJ, Osterhaus AD, Poelen M, Van Rooijen N, Claassen E: Antigen detection in vivo after immunization with different presentation forms of rabies virus antigen, II. Cellular, but not humoral, systemic immune responses against rabies virus immune-stimulating complexes are macrophage dependent. Immunology 94:455–460, 1998
Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, Chevailler A, Mouthon L, Weill B, Bruneval P, Kazatchkine MD, Kaveri SV: Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood 101:758–765, 2003
Schiff RI, Rudd C: Alterations in the half-life and clearance of IgG during therapy with intravenous gamma-globulin in 16 patients with severe primary humoral immunodeficiency. J Clin Immunol 6:256–264, 1986
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brinkman, D.M.C., Zijde, C.M.JV.D., Dam, M.M.T. et al. Vaccination with Rabies to Study the Humoral and Cellular Immune Response to a T-Cell Dependent Neoantigen in Man. J Clin Immunol 23, 528–538 (2003). https://doi.org/10.1023/B:JOCI.0000010429.36461.6b
Issue Date:
DOI: https://doi.org/10.1023/B:JOCI.0000010429.36461.6b