Abstract
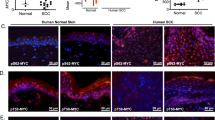
Studies assessed if the serine/threonine protein phosphatase-2A (PP-2A) maintains cytoskeletal integrity of normal keratinocytes and if this differs in malignant cells. Murine and human keratinocyte cell lines contained more PP-2A activity than did the murine SCC VII/SF squamous cell carcinoma cells or primary cultures of human head and neck squamous cell carcinoma (HNSCC) cells. Since tyrosine phosphorylation of the focal adhesion proteins paxillin and FAK is indicative of more stable focal adhesions, cells were immunostained for phosphotyrosine plus either paxillin or FAK, and then examined by confocal microscopy. In non-malignant keratinocytes, phosphotyrosine staining co-localized with paxillin and FAK. This co-localization occurred at the cell periphery in a pattern resembling focal adhesions. In contrast, the co-localization of phosphotyrosine with either paxillin or FAK along the cell periphery was almost absent in the SCC cells or in keratinocytes that were treated with okadaic acid to inhibit PP-2A activity. Consistent with this was a rounded cellular morphology with less extended processes as compared to control keratinocytes. These studies indicate PP-2A maintains the organization and tyrosine-phosphorylated state of the focal adhesion proteins FAK and paxillin, and that the loss of PP-2A activity results in a loss of cytoskeletal organization, as is seen in SCC.
Similar content being viewed by others
References
Lang SH, Hyde C, Reid IN et al. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate 2002; 52: 253–63.
Vadnais J, Nault G, Daher Z et al. Autocrine activation of the HGF-R/ Met tyrosine kinase induces tumor cell motility by regulating pseudopodial protrusion. J Biol Chem 2002
Otto T, Lummen G, Be A et al. Tumor cell motility. A novel therapeutic target in bladder carcinoma, experimental and clinical results. Adv Exp Med Biol 1999; 462: 469–76.
Cary LA Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front Biosci 1999; 4: D102–D113.
Greenwood JA, Theibert AB, Prestwich GD, Murphy-Ullrich JE. Restructuring of focal adhesion plaques by PI 3-kinase. Regulation by PtdIns (3,4,5)-p(3) binding to á-actinin. J Cell Biol 2000; 150: 627–42.
Ding Q, Gladson CL, Guidry CR et al. Extracellular FGF-1 inhibits cytoskeletal organization and promotes fibroblast motility. Growth Factors 2000; 18: 93–107.
Yu CF, Sanders MA, Basson MD. Human caco-2 motility redistributes FAK and paxillin and activates p38 MAPK in a matrix-dependent manner. Am J Physiol Gastrointest Liver Physiol 2000; 278: G952–G966.
Pokorna E, Jordan PW, O'Neill CH et al. Actin cytoskeleton and motility in rat sarcoma cell populations with different metastatic potential. Cell Motil Cytoskel 1994; 28: 25–33.
Lakshmi MS, Parker C, Sherbet GV. Metastasis and associated MTS1 and NM23 genes affect tubulin polymerisation in B16 melanomas: a possible mechanism of their regulation of metastatic behaviour of tumours. Anticancer Res 1993; 13: 299–303.
Kornberg LJ. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck 1998; 20: 745–52.
Reiske HR, Kao SC, Cary LA et al. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem 1999; 274: 12361–6.
Bang OS, Kim EJ, Chung JG. Association of focal adhesion kinase with fibronectin and paxillin is required for precartilage condensation of chick mesenchymal cells. Biochem Biophys Res Commun 2000; 278: 522–9.
Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol 2000; 2: E231–E236.
Yu DH, Qu CK, Henegariu O et al. Proteintyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem 1998; 273: 21125–31.
Young MRI, Liu SW, Meisinger J. Differences in association of the serine/threonine protein phosphatase PP-2A with microtubules of metastatic and nonmetastatic tumor cells. Clin Exp Metast 2001; 18: 407–13.
Jackson JL Young MR. Protein phosphatase-2A modulates the serine and tyrosine phosphorylation of paxillin in Lewis lung carcinoma tumor variants. Clin Exp Metast 2002; 19: 409–15.
Young MRI, Liu SW, Meisinger J. Protein phosphatase-2A restricts migration of Lewis lung carcinoma cells by modulating the phos-phorylation of focal adhesion proteins. Int J Cancer 2003; 103: 38–44.
Meisinger J, Patel S, Vellody K et al. Protein phosphatase-2A asso-ciation with microtubules and its role in restricting the invasiveness of human head and neck squamous cell carcinoma cells. Cancer Lett 1997; 111: 87–95.
Lozano Y, Taitz A, Petruzzelli GJ et al. Prostaglandin E 2-protein kinase A signaling and protein phosphatases-1 and-2A regulate human head and neck squamous cell carcinoma motility, adherence to extracellular matrix components, and cytoskeletal organization. Prostaglandins 1996; 51: 35–48.
Favre B, Turowski P, Hemmings BA. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem 1997; 272: 13856–13863.
Hardie DG, Haystead AJ, Sim ATR. Use of okadaic acid to inhibit protein phosphatases in intact cells. Methods Enzymol 1991; 201: 469–76.
Kabir-Salmani M, Shiokawa S, Akimoto Y et al. Characterization of morphological and cytoskeletal changes in trophoblast cells induced by insulin-like growth factor-I. J Clin Endocrinol Metab 2002; 87: 5751–9.
Romer LH, Burridge K, Turner CE. Signaling between the extracel-lular matrix and the cytoskeleton: Tyrosine phosphorylation and focal adhesion assembly. Cold Spring Harb Symp Quant Biol 1992; 57: 193–202.
Han JD Rubin CS. Regulation of cytoskeleton organization and paxillin dephosphorylation by cAMP. Studies on murine Y1 adrenal cells. J Biol Chem 1996; 271: 29211–5.
Vadlamudi R, Adam L, Talukder A et al. Serine phosphorylation of paxillin by heregulin-â 1: Role of p38 mitogen activated protein kinase. Oncogene 1999; 18: 7253–64.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romashko, A.A., Young, M.R.I. Protein phosphatase-2A maintains focal adhesion complexes in keratinocytes and the loss of this regulation in squamous cell carcinomas. Clin Exp Metastasis 21, 371–379 (2004). https://doi.org/10.1023/B:CLIN.0000046178.08043.f8
Issue Date:
DOI: https://doi.org/10.1023/B:CLIN.0000046178.08043.f8