Abstract
Purpose. To investigate the influence of astrocytes on P-glycoprotein (Pgp) expression and intracellular accumulation of Pgp substrates, separate from their net transcellular transport across the blood-brain barrier (BBB).
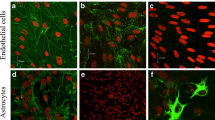
Methods. An in vitroBBB model was used, comprising of brain capillary endothelial cells (BCEC) monolayers or BCEC co-cultured with astrocytes.
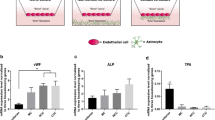
Results. BCEC+astrocyte co-cultures seemed to express a higher level of Pgp compared to BCEC monolayers. Inhibition of Pgp results in an increased intracellular accumulation of Pgp substrates in both BCEC monolayers and BCEC+astrocyte co-cultures, and increased the sensitivity for vinblastine mediated disruption of the in vitro BBB (called the vinblastine exclusion assay). BCEC monolayers were more sensitive to vinblastine mediated disruption compared to BCEC+astrocyte co-cultures. In the latter, but not in BCEC monolayers, an inhibitable polar transport of Pgp substrates was only found from the brain to the blood side of the filter.
Conclusions. Astrocytes increase the functional expression of Pgp in our in vitroBBB model. These results also illustrate that an important role for Pgp on the BBB is to protect the barrier against intracellular accumulation of cytotoxic BBB disrupting compounds.
Similar content being viewed by others
REFERENCES
C. Cordon-Cardo, J. P. O'Brien, D. Casals, L. Rittman-Grauer, J. L. Biedler, M. R. Melamed, and J. R. Bertino. Multidrugresistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. U.S.A. 86:695–698 (1989).
A. Tsuji, T. Terasaki, Y. Takabatake, Y. Tenda, I. Tamai, T. Yamashima, S. Moritani, T. Tsuruo, and J. Yamashita. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci. 51:1427–1437 (1992).
L. Fenart, V. Buee-Scherrer, L. Descamps, C. Duhem, M. G. Poullain, R. Cecchelli, and M. P. Dehouck. Inhibition of P-glycoprotein: rapid assessment of its implication in blood-brain barrier integrity and drug transport to the brain by an in vitro model of the blood-brain barrier. Pharm. Res. 15:993–1000 (1998).
D. J. Begley, D. Lechardeur, Z. D. Chen, C. Rollinson, M. Bardoul, F. Roux, D. Scherman, and N. J. Abbott. Functional expression of P-glycoprotein in an immortalised cell line of rat brain endothelial cells, RBE4. J. Neurochem 67:988–995 (1996).
R. C. Janzer and M. C. Raff. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325:253–257 (1987).
W. M. Pardridge, P. L. Golden, Y. S. Kang, and U. Bickel. Brain microvascular and astrocyte localization of P-glycoprotein. J. Neurochem. 68:1278–1285 (1997).
B. Joly, V. Lecureur, C. Puozzo, A. Guillouzo, and O. Fardel. Involvement of P-glycoprotein in an in vitro blood-brain barrier model. Int. J. Oncology 9:1029–1033 (1996).
A. Seelig. A general pattern for substrate recognition by P-glycoprotein. Eur. J. Biochem. 251:252–261 (1998).
I. C. J. van der Sandt, M. C. Blom-Roosemalen, A. G. de Boer, and D. D. Breimer. Specificity of doxorubicin versus thodamine-123 in assessing P-glycoprotein functionality in the LLC-PK1, LLC-PK1:MDR1 and Caco-2 cell lines. Eur. J. Pharm. Sci. (in press).
S. Nag. Role of the endothelial cytoskeleton in blood-brain-barrier permeability to protein. Acta Neuropathol. Berl. 90:454–460 (1995).
L. L. Mitic and J. M. Anderson. Molecular architecture of tight junctions. Annu. Rev. Physiol. 60:121–142 (1998).
D. Lechardeur and D. Scherman. Functional expression of the P-glycoprotein mdr in primary cultures of bovine cerebral capillary endothelial cells. Cell Biol. Toxicol. 11:283–293 (1995).
E. Beaulieu, M. Demeule, J. F. Pouliot, D. A. Averill Bates, G. F. Murphy, and R. Beliveau. P-glycoprotein of blood brain barrier: Cross-reactivity of Mab C219 with a 190 kDa protein in bovine and rat isolated brain capillaries. Biochim. Biophys. Acta 1233: 27–32 (1995).
U. Bommhardt, J. C. Cerottini, and H. R. MacDonald. Heterogeneity in P-glycoprotein (multidrug resistance) activity among murine peripheral T cells: Correlation with surface phenotype and effector function. Eur. J. Immunol. 24:2974–2981 (1994).
J. L. Madara. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 60:143–159 (1998).
C. Mouchard-Delmas, B. Gourdier, R. Vistelle, and M. Wiczewski. Modification of the blood-brain barrier permeability by vinorelbine: Effect of intracarotid infusion compared with intravenous infusion. Anticancer Drugs 7:213–219 (1996).
V. A. Levin. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 23:682–684 (1980).
A. H. Schinkel, E. Wagenaar, C. A. Mol, and L. van Deemter. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 97:2517–2524 (1996).
D. Leveque and F. Jehl. P-glycoprotein and pharmacokinetics. Anticancer Res. 15:331–336 (1995).
E. C. de Lange, G. de Bock, A. H. Schinkel, A. G. de Boer, and D. D. Breimer. BBB transport and P-glycoprotein functionality using MDR1A (-/-) and wild-type mice. Total brain versus microdialysis concentration profiles of rhodamine-123. Pharm. Res. 15:1657–1665 (1998).
O. C. Meijer, E. C. de Lange, D. D. Breimer, A. G. de Boer, J. O. Workel, and E. R. de Kloet. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology 139:1789–1793 (1998).
M. K. Spigelman, R. A. Zappulla, J. Johnson, S. J. Goldsmith, L. I. Malis, and J. F. Holland. Etoposide-induced blood-brain barrier disruption. Effect of drug compared with that of solvents. J. Neurosurg. 61:674–678 (1984).
L. A. MacDonell, P. E. Potter, and R. A. Leslie. Localized changes in blood-brain barrier permeability following the administration of antineoplastic drugs. Cancer Res. 38:2930–2934 (1978).
A. M. Yahanda, K. M. Alder, G. A. Fisher, N. A. Brophy, J. Halsey, R. I. Hardy, M. P. Gosland, B. L. Lum, and B. I. Sikic. Phase I trial of etoposide with cyclosporine as a modulator of multidrug resistance. J. Clin. Oncol. 10:1624–1634 (1992).
N. L. Bartlett, B. L. Lum, G. A. Fisher, N. A. Brophy, M. N. Ehsan, J. Halsey, and B. I. Sikic. Phase I trial of doxorubicin with cyclosporine as a modulator of multidrug resistance. J. Clin. Oncol. 12:835–842 (1994).
B. L. Lum and M. P. Gosland. MDR expression in normal tissues. Pharmacologic implications for the clinical use of P-glycoprotein inhibitors. Hematol. Oncol. Clin. North Am. 9:319–336 (1995).
J. Wijnholds, G. L. Scheffer, M. van der Valk, P. van der Valk, J. H. Beijnen, R. J. Scheper, and P. Borst. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J. Exp. Med. 188: 797–808 (1998).
M. Cereijido, J. Valdes, L. Shoshani, and R. G. Contreras. Role of tight junctions in establishing and maintaining cell polarity. Annu. Rev. Physiol. 60:161–177 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gaillard, P.J., van der Sandt, I.C.J., Voorwinden, L.H. et al. Astrocytes Increase the Functional Expression of P-Glycoprotein in an In Vitro Model of The Blood-Brain Barrier. Pharm Res 17, 1198–1205 (2000). https://doi.org/10.1023/A:1026406528530
Issue Date:
DOI: https://doi.org/10.1023/A:1026406528530