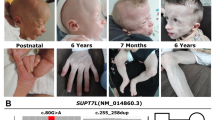
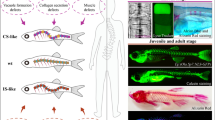
Abstract
The name glypican identifies a family of heparan sulfate proteoglycans that are linked to the cell surface by a glycosylphosphatidylinositol anchor. Members of this family have been identified in Drosophila, zebrafish, and mammals. The interest in the study of glypicans has increased in the last few years as a result of the discovery that the glypican-3 gene (GPC-3) is mutated in an overgrowth and dysmorphic syndrome. Despite the increased interest, our knowledge about the function of glypicans is still limited, since the molecular basis for the role of glypican-3 in the regulation of body size remains unknown. The in vivo manipulation of glypican expression in lower organisms, however, has demonstrated that these proteoglycans can modulate cellular responses to Wnts and bone morphogenetic factors . Future studies should investigate whether the phenotype of GPC-3-deficient individuals is also due to altered modulation of cellular responses to these factors. Published in 2003.
Similar content being viewed by others
References
Filmus J, Song HH, in Glypicans, edited by Iozzo RV. Proteoglycans, New York, Marcel Dekker, pp. 161–76.
Filmus J, Selleck SB, Glypicans: Proteoglycans with a surprise, J Clin Invest 108, 497–501 (2001).
Veugelers M, De Cat B, Ceulemans H, Bruystens AM, Coomans C, Durr J, Vermeesch J, Marynen P, David G, Glypican-6, a new member of the glypican family of cell surface proteoglycans, J Biol Chem 274, 26968–77 (1999).
Saunders S, Paine-Saunders S, Lander AD, Expression of the cell surface proteoglycan glypican-5 is developmentally regulated in kidney, limb, and brain, Dev Biol 190, 78–93 (1997).
David G, Lories V, Decock B, Marynen P, Cassiman J, Van Den Berghe H, Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts, J Cell Biol 111, 3165–76 (1990).
Stipp CS, Litwac ED, Lander AD, Cerebroglycan: An integral membrane heparan sulfate proteoglycan that is unique to the developing nervous system and expressed specifically during neuronal differentiation, J Cell Biol 124, 149–60 (1994).
Filmus J, Church J, Buick RN, Isolation of a cDNA corresponding to a developmentally regulated transcript in rat intestine, Mol Cell Biol 8, 4243–9 (1988).
Watanabe K, Yamada H, Yamaguchi Y, K-glypican: A novel GPIlinked heparan sulfate proteoglycan that is highly expressed in developing brain and kidney, J Cell Biol 130, 1207–18 (1995).
Veugelers M, Vermeesch J, Reekmans G, Steinfeld R, Marynen P, David G, Characterization of glypican-5 and chromosomal localization of human GPC5, a new member of the glypican gene family, Genomics 40, 24–30 (1997).
Paine-Saunders S, Viviano BL, Saunders S, GPC6, a novel member of the glypican gene family, encodes a product structurally related to GPC4 and is colocalized with GPC5 on human chromosome 13, Genomics 57, 455–8 (1999).
Nakato H, Futch TA, Selleck SB, The division abnormally delayed (dally) gene: A putative integral membrane proteoglycan required for cell division patterning during postembryonic development of the nervous system in Drosophila, Development 121, 3687–702 (1995).
Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N, Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless, Development 128, 87–94 (2001).
Topczewsky J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L, The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension, Dev Cell 1, 251–64 (2001).
Litwack ED, Stipp CS, Kumbasar A, Lander AD, Neuronal expression of glypican, a cell surface glycosylphosphatidylinositolanchored heparan sulfate proteoglycan, in the adult rat nervous system, J Neurosci 14, 3713–24 (1994).
Ivins JK, Litwack ED, Kumbasar A, Stipp CS, Lander AD, Cerebroglycan, a developmentally regulated cell-surface heparan sulfate proteoglycan, is expressed on developing axons and growth cones, Dev Biol 184, 320–32 (1997).
Pellegrini M, Pilia G, Pantano S, Lucchini F, Uda M, Fumi M, Cao A, Schlessinger D, Forabosco A, Gpc3 expression correlates with the phenotype of the Simpson-Golabi-Behmel syndrome, Dev Dyn 213, 431–9 (1998).
Li M, Choo B, Wong Z-M, Filmus J, Buick RN, Expression of OCI-5/Glypican 3 during intestinal morphogenesis: Regulation by cell shape in intestinal epithelial cells, Exp Cell Res 235, 3–12 (1997).
Veugelers M, Vermeesch J, Watanabe K, Yamaguchi Y, Marynen P, David G, GPC4, the gene for human K-glypican, flanks GPC3 on Xq26: Deletion of the GPC3-GPC4 gene cluster in one family with Simpson-Golabi-Behmel syndrome, Genomics 53, 1–11 (1998).
Siebertz B, Stocker G, Drzeniek Z, Handt S, Just U, Haubeck H-D, Expression of glypican-4 in haematopoietic-progenitor and bone-marrow-stromal cells, Biochem J 344, 937–43 (1999).
Fujise M, Izumi S, Selleck SB, Nakato H, Regulation of dally, an integral membrane proteoglycan, and its function during adult sensory organ formation of Drosophila, Dev Biol 235, 433–48 (2001).
Lin X, Perrimon N, Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signaling, Nature 400, 281–4 (1999).
Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, Siegfried E, Stam L, Selleck SB, The cell-surface proteoglycan Dally regulates Wingless signaling in Drosophila, Nature 400, 276–80 (1999).
Jackson SM, Nakato H, Sugiura M, Jannuzi A, Oakes R, Kaluza V, Golden C, Selleck SB, dally, a Drosophila glypican, controls celular responses to the TGF-beta-related morphogen Dpp, Development 124, 4113–20 (1997).
Baeg GH, Perrimon N, Functional binding of secreted molecules to heparan sulfate proteoglycans in Drosophila, Curr Opin Cell Biol 12, 575–80 (2000).
Binari RC, Staveley BE, Johnson WA, Godovarti R, Sasisekharan R, Manoukian AS, Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling, Development 124, 2623–32 (1997).
Haerry TE, Heslip TR, Marsh JL, O'Connor MB, Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila, Development 124, 3055–64 (1997).
Reichsman F, Smith L, Cumberledge S, Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction, J Cell Biol 135, 819–27 (1996).
Niehrs C, Solving a sticky problem, Nature 413, 787–8 (2001).
Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D, Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome, Nature Genet 12, 241–7 (1996).
Neri G, Gurrieri F, Zanni G, Lin A, Clinical and molecular aspects of the Simpson-Golabi-Behmel syndrome, Am J Med Genet 79, 279–83 (1998).
Hughes-Benzie RM, Pilia G, Xuan JY, Hunter AGW, Chen E, Golabi M, Hurst JA, Kobori J, Marymee K, Pagon RA, Punnett HH, Schelley S, Tolmie JL, Wohlferd MM, Grossman T, Schlessinger D, Mackenzie AE, Simpson-Golabi-Behmel syndrome: Genotype/phenotype analysis of 18 affected males from 7 unrelated families, Am J Med Genet 66, 227–34 (1996).
Garganta CL, Bodurtha JN, Report of another family with Simpson-Golabi-Behmel syndrome and a review of the literature, Am J Med Genet 44, 129–35 (1992).
Behmel A, Plochl E, Rosenkranz W, A new X-linked dysplasia gigantism syndrome: Identical with the Simpson dysplasia syndrome? Hum Genet 67, 409–13 (1984).
Golabi M, Rosen L, A new X-linked mental retardation overgrowth syndrome, Am J Med Genet 17, 345–58 (1984).
Lapunzina P, Badia I, Galoppo C, De Matteo E, Silberman P, Tello A, Grichener J, Hughes-Benzie R, A patient with Simson-Golabi-Behmel syndrome and hepatocellular carcinoma, J Med Genet 35, 153–6 (1998).
Xuan JY, Hughes-Benzie RM, Mackenzie AE, A small interstitial deletion in the GPC3 gene causes Simpson-Golabi-Behmel syndrome in a Dutch-Canadian family, J Med Genet 36, 57–8 (1999).
Hughes-Benzie RM, Hunter AGW, Allanson JE, Mackenzie AE, Simson-Golabi-Behmel syndrome associated with renal dysplasia and embryonal tumor: Localization of the gene to Xqcen-q21, Am J Med Genet 43, 428–35 (1992).
Li M, Shuman C, Fei YL, Cutiongco E, Bender HA, Stevens C, Wilkins-Haug L, Day-Salvatore D, Yong SL, Geraghty MT, Squire JA, Weksberg R, GPC3 mutation analysis in a spectrum of patients with overgrowth expands the phenotype of Simpson-Golabi-Behmel syndrome, Am J Med Genet 102, 161–8 (2001).
Cano-Gauci DF, Song H, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, Sedlackova L, Tanswell AK, Mak TW, Yeger H, Lockwood GA, Rosenblum N, Filmus J, Glypican-3-deficient mice exhibit the overgrowth and renal abnormalities typical of the Simpson-Golabi-Behmel syndrome, J Cell Biol 146, 255–64 (1999).
Paine-Saunders S, Viviano BL, Zupicich J, Skarnes WC, Saunders S, Glypican-3 controls cellular responses to Bmp4 in limb patterning and skeletal development, Dev Biol 225, 179–87 (2000).
Chiao E, Fisher P, Crisponi L, Deiana M, Dragatsis I, Schlessinger D, Pilia G, Efstratiadis A, Overgrowth of a mouse model of the Simpson-Golabi-Behmel syndrome is independent of IGF signaling, Dev Biol 243, 185–206 (2002).
Duenas Gonzales A, Kaya M, Shi W, Song H, Testa JR, Penn LZ, Filmus J, OCI-5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell line-specific manner, J Cell Biol 141, 1407–14 (1998).
Weng EY, Mortier GR, Graham JM, Beckwith-Wiedemann syndrome, Clin Pediat 34, 317–26 (1995).
Weksberg R, Squire JA, Molecular biology of Beckwith-Wiedemann syndrome, Med Pediatric Oncol 27, 462–9 (1997).
Weksberg R, Squire JA, Templeton DM, Glypicans: A growing trend, Nature Genet 12, 225–7 (1996).
Eggenschwiler J, Ludwig T, Fisher P, Leighton PA, Tilghman SM, Efstratiadis A, Mouse mutant embryos overexpressing IGFII exhibit phenotypic features of the Beckwith-Wiedemann and Simpson-Golabi-Behmel syndromes, Genes & Develop 11, 3128–42 (1997).
Song HH, Shi W, Filmus J, OCI-5/rat glypican-3 binds to fibroblast growth factor-2 but not to insulin-like growth factor-2, J Biol Chem 272, 7574–7 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filmus, J. The contribution of in vivo manipulation of gene expression to the understanding of the function of glypicans. Glycoconj J 19, 319–323 (2002). https://doi.org/10.1023/A:1025312819804
Issue Date:
DOI: https://doi.org/10.1023/A:1025312819804