Abstract
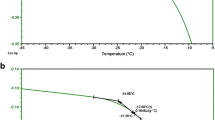
Purpose. To examine whether the glass transition temperature (Tg) of freeze-dried formulations containing polymer excipients can be accurately predicted by molecular dynamics simulation using software currently available on the market. Molecular dynamics simulations were carried out for isomaltodecaose, a fragment of dextran, and α-glucose, the repeated unit of dextran, in the presence or absence of water molecules. Estimated values of Tg were compared with experimental values obtained by differential scanning calorimetry (DSC).
Methods. Isothermal-isobaric molecular dynamics simulations (NPTMD) and isothermal molecular dynamics simulations at a constant volume (NVTMD) were carried out using the software package DISCOVER (Material Studio) with the Polymer Consortium Force Field. Mean-squared displacement and radial distribution function were calculated.
Results. NVTMD using the values of density obtained by NPTMD provided the diffusivity of glucose-ring oxygen and water oxygen in amorphous α-glucose and isomaltodecaose, which exhibited a discontinuity in temperature dependence due to glass transition. Tg was estimated to be approximately 400K and 500K for pure amorphous α-glucose and isomaltodecaose, respectively, and in the presence of one water molecule per glucose unit, Tg was 340K and 360K, respectively. Estimated Tg values were higher than experimentally determined values because of the very fast cooling rates in the simulations. However, decreases in Tg on hydration and increases in Tg associated with larger fragment size could be demonstrated.
Conclusions. The results indicate that molecular dynamics simulation is a useful method for investigating the effects of hydration and molecular weight on the Tg of lyophilized formulations containing polymer excipients, although the relationship between cooling rates and Tg must first be elucidated to predict Tg vales observed by DSC measurement. January 16
Similar content being viewed by others
References
J. Han, R. H. Gee, and R. H. Boyd. Glass transition temperatures of polymers from molecular dynamics simulations. Macromolecules 27:7781–7784 (1994).
M. Tsige and P. L. Taylor. Simulation study of the glass transition temperature in poly(methyl methacrylate). Phys. Rev. E. 65:1–8 (2002).
F. A. Momany and J. L. Willett. Molecular dynamics calculations on amylose fragments. I. Glass transition temperatures of maltodecaose at 1, 5, 10, and 15.8% hydration. Biopolymers 63:99–110 (2002).
P. B. Conrad and J. J. de Pablo. Computer simulation of the cryoprotectant disaccharide α,α-trehalose in aqueous solution. J. Phys. Chem. A 103:4049–4055 (1999).
N. C. Ekdawi-Sever, P. B. Conrad, and J. J. de Pablo. Molecular simulation of sucrose solutions near the glass transition temperature. J. Phys. Chem. A. 105:734–742 (2001).
E. R. Caffarena and J. R. Grigera. Hydration of glucose in the rubbery and glassy states studied by molecular dynamics simulation. Carbohydrate Res. 315:63–69 (1999).
E. R. Caffarena and J. R. Grigera. Glass transiton in aqueous solutions of glucose. Molecular dynamics simulation. Carbohydrate Res. 300:51–57 (1997).
C. J. Roberts and P. G. Debenedetti. Structure and dynamics in concentrated, amorphous carbohydrate-water systems by molecular dynamics simulation. J. Phys. Chem. B 103:7308–7318 (1999).
S. Yoshioka, Y. Aso, and S. Kojima. Dependence of the molecular mobility and protein stability of freeze-dried γ-globulin formulations on the molecular weight of dextran. Pharm. Res. 14:736–741 (1997).
F. Franks. Freeze drying: From empiricism to predictability. Cryo-Letters 11:93–110 (1990).
S. J. Prestrelski, K. A. Pikal, and T. Arakawa. Optimization of lyophilization conditions for recombinant human interleukin-2 by dried-state conformational analysis using fourier-transform infrared spectroscopy. Pharm. Res. 12:1250–1259 (1995).
C. T. Moynihan, A. J. Easteal, and J. Wilder. Dependence of the glass transition temperature on heating and cooling rate. J. Phys. Chem. 78:2673–2677 (1974).
V. Andronis and G. Zografi. The molecular mobility of supercooled amorphous indomethacin as a function of temperature and relative humidity. Pharm. Res. 15:835–842 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshioka, S., Aso, Y. & Kojima, S. Prediction of Glass Transition Temperature of Freeze-Dried Formulations by Molecular Dynamics Simulation. Pharm Res 20, 873–878 (2003). https://doi.org/10.1023/A:1023831102203
Issue Date:
DOI: https://doi.org/10.1023/A:1023831102203