Abstract
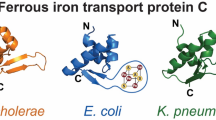
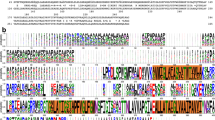
Many gram-negative bacteria produce and excrete siderophores, which complex iron with high affinity in the environment. The ferric siderophore complexes are transported across the outer membrane by receptor proteins. This process requires energy and is TonB dependent and must involve conformational changes in the receptor proteins to allow the transport of the ferric siderophores from the extracellular binding site to the periplasm. There is a large variety in the structures, molecular weights and charges among the siderophores. It was therefore realized that when the sequences of the many different receptor proteins were compared, simultaneously, all identities and close similarities, found in this manner, could only be due to residues involved in the conformational changes and transport mechanism, common to all the proteins, and not be due to the specificity of ligand recognition. Once the crystal structures of FepA, FhuA and FecA became available, it was immediately clear that the sequence similarities which were found in the simultaneous alignment, were all localized in a few structural domains, which are identical in the three structures and can therefore be expected to be maintained in all the proteins in this family. One of these domains, tentatively named the lock region, consists of 10 residues with a central quadrupole formed by two arginines and two glutamates, from the plug region and the beta barrel. We mutated several of these residues in FepA. All showed normal binding in quantitative binding studies. Some showed normal transport as well, however, the majority showed moderate to severe defective transport with ferric enterobactin. The results therefore show the validity of the hypothesis that the simultaneous sequence alignment will select the residues involved in the transport function of the receptor proteins. In addition the results allow to relate the severity of the transport deficiency to be correlated with the structure of the lock region while it is also possible to propose a function of this region in the conformational changes of the protein during the transport of the ligand from the binding site to the periplasm.
Similar content being viewed by others
References
Archibald F. 1983 Lactobacillus plantarum, an organism not requiring iron FEMS Microbiol Lett 19, 29–32.
Bagg A, Neilands JB. 1987 Molecular mechanism of siderophoremediated iron assimilation. Microbiol Rev 51, 509–518.
Barnard TJ, Watson ME Jr, McIntosh MA. 2001 Mutations in the Escherichia coli receptor FepA reveal residues involved in ligand binding and transport. Mol Microbiol 41, 527–536.
Braun V, Killmann H. 1999 Bacterial solutions to the iron supply problem. Trends Biochem Sci 24, 104–109.
Braun V, Hantke K, Köster W. 1998 Bacterial iron transport: mechanisms, genetics, and regulation. In: Sigel A and Sigel H, eds. Metal ions in biological systems, Vol. 35. Iron transport and storage in microorganisms, plants, and animals. New York, N.Y.: Marcel Dekker; pp. 67–145.
Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. 1999 Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol 6, 56–63.
Cadieux N, Kadner RJ. 1999 Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc Natl Acad Sci USA 96(19), 10673–10678.
Clarke TE, Ku SY, Dougan DR, Vogel HJ, Tari LW. 2000 The structure of the ferric siderophore binding protein FhuD complexed with gallichrome. Nat Struct Biol 7(4), 287–291.
Clarke TE, Braun V, Winkelmann G, Tari LW, Vogel HJ. 2002 X-ray crystallographic structures of the Escherichia coli periplasmic protein FhuD bound to hydroxamate-type siderophores and the antibiotic albomycin. J Biol Chem 277(16), 13966–13972.
Ecker DJ, Matzanke BF, Raymond KN. 1986 Recognition and transport of ferric enterobactin in Escherichia coli. J Bacteriol 167, 666–673.
Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W. 1998 Siderophore mediated iron transport: Crystal structure of FhuA with bound lipopolysaccharide. Science 282, 2215–2220.
Ferguson AD, Chakraborty R, Smith BS, Esser L, van der Helm D, Deisenhofe, J. (2002) Structural basis of gating by the outer membrane Transporter FecA. Science 295, 1715–1719.
Guiseppi JD, Fridovich I. 1982 Oxygen toxicity in Streptococcus sanguis. J Biol Chem 257, 4046–4051.
Howard SP, Herrmann C, Stratilo CW, Braun V. 2001 In vivo synthesis of the periplasmic domain of TonB inhibits transport through the FecA and FhuA iron siderophore transporters of Escherichia coli. J Bacteriol 183(20), 5885–5895.
Hutchison CA, Phillips S, Edgell MH, Gillam S, Jahnke P, Smith M. 1978 Mutagenesis at a specific position in a DNA sequence. J Biol Chem 253, 6551–6560.
Larsen RA, Foster-Hartnett D, McIntosh MA, Postle K. 1997 Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J Bacteriol 179, 3213–3221.
Larsen RA, Thomas M G, Postle K. 1999 Proton motive force, ExbB, and ligand-bound FepA drive conformational changes in TonB. Mol Microbiol 31, 1809–1824.
Lemke EA. 2001 Biophysical characterization of FepA mutants. M.S. Thesis, University of Oklahoma, Norman, U.S.A.
Locher K P, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbusch JP, Moras D. 1998 Trans membrane signaling across the ligand-gated FhuA receptor: Crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95, 771–778.
Miller JH. 1972 Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Moeck GS, Coulton JW. 1998 TonB dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol Microbiol 28, 675–681.
Moeck GS, Letellier L. 2001 Characterization of in vitro interactions between a truncated TonB protein from Escherichia coli and the outer membrane receptors FhuA and FepA. J Bacteriol 183, 2755–2764.
Neidhardt FC, Bloch PL, Smith DF. 1974 Culture medium for enterobacteria. J Bacteriol 119, 736–747.
Newton SM, Allen JS, Cao Z, Qi Z, Jiang X, Sprencel C, Igo JD, Foster SB, Payne MA, Klebba PE. 1997 Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc Natl Acad Sci USA 94, 4560–4565.
Newton SMC, Igo JD, Scott D, Klebba PE. 1999 Effects of loop deletions on the binding and transport of ferric enterobactin by FepA. Mol Microbiol 32, 1153–1165.
Payne MA, Igo JD, Cao Z, Foster SB, Newton SMC, Klebba PE. 1997 Biphasic binding kinetics between FepA and its ligands. J Bacteriol 272, 21950–21955.
Rutz JM, Liu J, Lyons JA, Goranson J, Armstrong SK, McIntosh MA et al. 1992 Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science 258, 471–475.
Schryvers AB, Stojiljkovic I. 1999 Iron acquisition systems in the pathogenic Neisseria. Mol Microbiol 32, 1117–1123.
Scott DC, Cao Z, Qi Z, Bauler M, Igo JD, Newton SMC, Klebba PE. 2001 Exchangeability of N termini in the ligand-gated porins of Escherichia coli. J Biol Chem 276, 13025–13033.
Shea CM, McIntosh MA. 1991 Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein dependent systems in Escherichia coli. Mol Microbiol 5, 1415–1428.
Skare JT, Ahmer BMM, Seachord CL, Darveau RP, Postle K. 1993 Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J Biol Chem 268, 16302–16308.
Sprencel C, Cao Z, Qi Z, Scott DC, Montague MA, Ivanoff N, Xu J, Raymond KM, Newton SMC, Klebba PE. 2000 Binding of ferric enterobactin by the Escherichia coli periplasmic protein FepB. J Bacteriol 182, 5359–5364.
Staudenmaier H, Brunhilde van H, Zahra Y, and Braun V. 1989 Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for Iron(III) dicitrate in Escherichia coli. J Bacteriol 171, 2626–2633.
Thompson JD, Higgins DG, Gibson TJ. 1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22, 4673–4680.
Thulasiraman P, Newton SMC, Xu J, Raymond KN, Mai C, Hall A, Montague MA, Klebba PE. 1998 Selectivity of ferric enterobactin binding and cooperativity of transport in gram-negative bacteria. J Bacteriol 180, 6689–6696.
van der Helm D. 1998 The physical chemistry of bacterial outermembrane siderophore receptor proteins. In: Sigel A and Sigel H., eds. Metal ions in biological systems, vol. 35. Iron transport and storage in microorganisms, plants, and animals. New York, N.Y.: Marcel Dekker; pp. 355–401
van der Helm D, Chakraborty R. 2001 Structures of siderophore receptors. In: Winkelman G., ed. Microbial Transport Systems. Weinheim, Germany: Wiley-VCH; pp. 261–288.
van der Helm D, Chakraborty R, Ferguson AD, Smith BS, Esser L, Deisenhofer J. 2002 Bipartite gating in the outer membrane protein FecA. Biochem Soc Trans 30, 708–710.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chakraborty, R., Lemke, E.A., Cao, Z. et al. Identification and mutational studies of conserved amino acids in the outer membrane receptor protein, FepA, which affect transport but not binding of ferric-enterobactin in Escherichia coli . Biometals 16, 507–518 (2003). https://doi.org/10.1023/A:1023485632520
Issue Date:
DOI: https://doi.org/10.1023/A:1023485632520