Abstract
Purpose. The purpose of this work was to determine the jejunal permeability of cyclosporin A (CsA) in humans and whether formulation variables modulate the effects of P-glycoprotein (P-gp) on the permeability of CsA in Caco-2 cells.
Methods. A solution containing CsA, phenylalanine, propranolol, polyethyleneglycol (PEG) 400, and PEG 4000 was perfused through a 10-cm jejunal segment in 12 subjects. Caco-2 transport studies were performed using previously reported methodology.
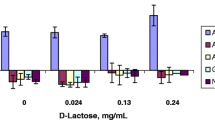
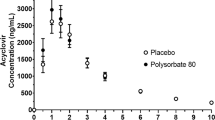
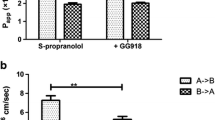
Results. The mean P eff (±SD) of CsA in humans was 1.65 (0.53). The mean permeabilities for phenylalanine, propranolol, and PEG 400 were 4.54 (2.39), 2.90 (1.28), and 0.83 (0.51) × 10-4 cm/s, respectively. The presence of surfactants significantly decreased the permeabilities of CsA in both directions in Caco-2 cells.
Conclusions. The results suggest that the effects of surfactants via micellar solubilization and inhibition of P-gp efflux on CsA transport in Caco-2 cells are significant. CsA can rightly be classified as a low solubility-high permeability Class II BCS drug and its highly variable absorption from Sandimmune® oral formulations is the result of poor dissolution characteristics.
Similar content being viewed by others
REFERENCES
G. Ismailos, C. Reppas, J. B. Dressman, and P. Macheras. Unusual solubility behaviour of cyclosporin A in aqueous media. J. Pharm. Pharmacol. 43:287-289 (1991).
J. Grevel, E. Nuesch, E. Abisch, and K. Kutz. Pharmacokinetics of oral cyclosporin A (Sandimmun) in healthy subjects. Eur. J. Clin. Pharmacol. 31:211-216 (1986).
S. K. Gupta and L. Z. Benet. Absorption kinetics of cyclosporine in healthy volunteers. Biopharm. Drug Dispos. 10:591-596 (1989).
A. Lindholm, S. Henricsson, M. Lind, and R. Dahlqvist. Intraindividual variability in the relative systemic availability of cyclosporin after oral dosing. Eur. J. Clin. Pharmacol. 34:461-464 (1988).
U. Christians and K. F. Sewing. Cyclosporin metabolism in transplant patients. Pharmacol. Ther. 57:291-345 (1993).
P. B. Watkins. Noninvasive tests of CYP3A enzymes. Pharmacogenetics 4:171-184 (1994).
J. H. Charuk, P. Y. Wong, and R. A. Reithmeier. Differential interaction of human renal P-glycoprotein with various metabolites and analogues of cyclosporin A. Am. J. Physiol. 269:F31-F39 (1995).
M. Lemaire, A. Fahr, and G. Maurer. Pharmacokinetics of cyclosporine: inter-and intra-individual variations and metabolic pathways. Transplant. Proc. 22:1110-1112 (1990).
A. Lindholm, M. Welsh, C. Alton, and B. D. Kahan. Demographic factors influencing cyclosporine pharmacokinetic parameters in patients with uremia: racial differences in bioavailability. Clin. Pharmacol. Ther. 52:359-371 (1992).
J. Drewe, C. Beglinger, and T. Kissel. The absorption site of cyclosporin in the human gastrointestinal tract. Br. J. Clin. Pharmacol. 33:39-43 (1992).
N. Takamatsu, L. S. Welage, N. M. Idkaidek, D.-Y. Liu, P. I.-D. Lee, Y. Hayashi, J. K. Rhie, H. Lennernas, J. L. Barnett, V. P. Shah, L. Lesko, and G. L. Amidon. Human intestinal permeability of piroxicam, propranolol, phenylalanine and PEG 400 determined by jejunal perfusion. Pharm. Res. 14:1127-1132 (1997).
N. Takamatsu, O.-N. Kim, L. S. Welage, N. M. Idkaidek, Y. Hayashi, J. L. Barnett, R. Yamomoto, E. Lipka, H. Lennernas, A. Hussain, L. Lesko, and G. L. Amidon. Human jejunal permeability of two polar drugs: Cimetidine and ranitidine. Pharm. Res. 18:742-744 (2001).
I. M. Menzies. Transmucosal passage of inert molecules in health and disease. In E. Skadhauge and K. Heintze (eds.), Intestinal Absorption and Secretion, MTP Press, Lancaster, Pennsylvania, 1983 pp. 527-543.
B. G. Munck. Intestinal absorption of amino acids. In L. F. Johnson (ed.), Physiology of the Gastrointestinal Tract, Raven Press, New York, 1981 pp. 1097-1122.
C. Hilgendorf, H. Spahn-Langguth, C. G. Regardh, E. Lipka, G. L. Amidon, and P. Langguth. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: Permeabilities via diffusion, inside-and outside-directed carrier-mediated transport. J. Pharm. Sci. 89:63-75 (2000).
X.-Y. Chu, G. P. Sanchez-Castano, K. Higaki, D.-M. Oh, C.-P. Hsu, and G. L. Amidon. Correlation between epithelial cell permeability of cephalexin and expression of intestinal oligopeptide transporter. J. Pharmacol. Exp. Ther. 299:575-582 (2001).
W. M. Awni and J. A. Maloney. Optimized high-performance liquid chromatographic method for the analysis of cyclosporine and three of its metabolites in blood and urine. J. Chromatogr. 425:233-236 (1998).
A. A. al-Angary, Y. M. el-Sayed, M. A. al-Meshal, M. M. al-Dardiri, and G. M. Mahrous. A sensitive high-performance liquid chromatographic analysis of propranolol in serum. J. Clin. Pharm. Ther. 16:93-101 (1991).
M. M. Nerurkar, P. S. Burton, and R. T. Borchardt. The use of surfactants to enhance permeability of peptides through Caco-2 cells by inhibition of apically polarized efflux system. Pharm. Res. 13:528-534 (1996).
M. M. Nerurkar, N. F. H. Ho, P. S. Burton, T. J. Vidmar, and R. T. Borchardt. Mechanistic roles of neutral surfactants on cocurrent polarized and passive membrane transport of a model peptide in Caco-2 cells. J. Pharm. Sci. 86:813-821 (1997).
F. Ingels, S. Deferme, E. Destexhe, M. Oth, G. Van den Mooter, and P. Augustijns. Simulated intestinal fluid as transport medium in the Caco-2 cell culture model. Int. J. Pharm. 232:183-192 (2002).
E. V. Batrakova, H.-Y. Han, V. Y. Alakhov, D. W. Miller, and A. V. Kabanov. Effects of Pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm. Res. 15:850-855 (1998).
I. Knemeyer, M. G. Wientjes, and J. L.-S. Au. Cremophor reduces paclitaxel penetration into bladder wall during intravesical treatment. Cancer Chemother. Pharmacol. 44:241-248 (1999).
S. Tamura, A. Ohike, R. Ibuki, G. L. Amidon, and S. Yamashita. Tacrolimus is a Class II low solubility-high permeability drug: The effect of P-glycoprotein efflux on regional permeability of tacrolimus in rats. J. Pharm. Sci. 91:719-729 (2002).
G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413-420 (1995).
S. K. Gupta, R. C. Manfro, S. J. Tomlanovich, J. G. Gambertoglio, M. R. Garovoy, and L. Z. Benet. Effect of food on the pharmacokinetics of cyclosporine in healthy subjects following oral and intravenous administration. J. Clin. Pharmacol. 30:643-653 (1990).
E. A. Mueller, J. M. Kovarik, J. B. van Bree, W. Tetzloff, J. Grevel, and K. Kutz. Improved dose linearity of cyclosporine pharmacokinetics from a microemulsion formulation. Pharm. Res. 11:301-304 (1994).
E. A. Mueller, D. Niese, and B. Mellein. Cyclosporine microemulsion formulation (Neoral) in transplantation: Pharmacokinetic/pharmacodynamic relationships. Transplant Proc. 30:1694-1696 (1998).
Z.-G. Gao, H.-G. Choi, H.-J. Shin, K.-M. Park, S.-J. Lim, K.-J. Hwang, and C.-K. Kim. Physicochemical characterization and evaluation of a microemulsion system for oral delivery of cyclosporin A. Int. J. Pharm. 161:75-86 (1998).
J. Guo, Q. Ping, and Y. Chen. Pharmacokinetic behavior of cyclosporin A in rabbits by oral administration of lecithin vesicle and sandimmun neoral. Int. J. Pharm. 216:17-21 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiu, YY., Higaki, K., Neudeck, B.L. et al. Human Jejunal Permeability of Cyclosporin A: Influence of Surfactants on P-Glycoprotein Efflux in Caco-2 Cells. Pharm Res 20, 749–756 (2003). https://doi.org/10.1023/A:1023481418576
Issue Date:
DOI: https://doi.org/10.1023/A:1023481418576