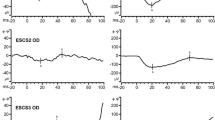
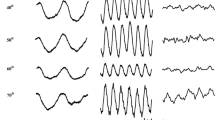
Abstract
Photoreceptor and post-receptoral function in children with congenital and acquired cone disorders was measured by full-field electroretinogram (ERG) and transient visual evoked potentials (VEPs). Subjects were five rod monochromats (RM), five with cone dystrophy (CD), and 30 controls. Patients were diagnosed by clinical findings, ERGs, and standard color vision tests. VEP stimuli were check reversals and color grating onsets that stimulated each photoreceptor type (L-, M-, or S-cones) or post-receptoral pathways (L–M, white/black). VEP signal-to-noise ratios (S/N) were calculated by Fourier analysis of VEP epochs. All RM patients showed extinguished cone ERGs. A near normal S-cone VEP was recorded from a blue-cone rod monochromat without any signal from the L- or M-cone stimuli. Two other RM patients were classified as incomplete RM based on a low-level VEP signal from either L- or M-cone stimuli. CD patients had mildly to severely reduced ERGs and VEPs were abnormal to all cone-isolating stimuli. The VEP S/N ratio was not significantly correlated with the amount of rod contrast in the color stimuli. Color VEPs provide an objective assessment of macular cone function in children with cone dysfunction syndromes that is more sensitive to residual central cone function than standard full-field ERGs. VEP techniques may be useful in the early detection of cone loss in children, especially in children who do not tolerate ERG testing.
Similar content being viewed by others
References
Krill AE, Deutman AF. Dominant macular degenerations: The cone dystrophies. Am J Ophthalmol 1972; 73: 352–369.
Goodman G, Ripps H, Siegel IM. Cone dysfunction syndromes. Arch Ophthalmol 1963; 70: 214–231.
Berson EL, Gouras P, Gunkel RD. Progressive cone degeneration, dominantly inherited. Arch Ophthalmol 1968; 80: 77–83.
Ripps H, Noble KG, Greenstein VC, Siegel IM, Carr RE. Progressive cone dystrophy. Ophthalmology. 1987; 94: 1401–1409.
Reichel E, Bruce AM, Sandberg MA, Berson EL. An electroretinographic and molecular genetic study of X-linked cone degeneration. Am J Ophthalmol 1989; 108: 540–547.
Keunen JE, van Everdingen JA, Went LN, Oosterhuis JA, van Norren D. Color matching and foveal densitometry in patients and carriers of an X-linked progressive cone dystrophy. Arch Ophthalmol 1990; 108: 1713–1719.
Nathans J, Maumenee IH, Zrenner E, et al. Genetic heterogeneity among blue-cone monochromats. Am J Hum Genet 1993; 53: 987–1000.
Kohl S, Marx T, Giddings I, et al. Total colourblindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cgMP-gated cation channel. Nat Genet 1998; 19: 257–259.
Kohl S, Baumann B, Broghammer M, et al. Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cgMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21. HumMol Genet 2000;9: 2107–2116.
Sundin OH, Yang JM, Li Y, et al. Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet 2000; 25: 289–293.
Fishman GA, Stone EM, Alexander KR, Gilbert LD, Derlacki DJ, Butler NS. Serine-27-phenylalanine mutation within the peripherin/RDS gene in a family with cone dystrophy. Ophthalmology1997; 104: 299–306.
Payne AM, Downes SM, Bessant DA, et al. A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21. Hum Mol Genet 1998; 7: 273–277.
Sokal I, Li N, Surgucheva I, et al. GCAP1 (Y99C) mutant is constitutively active in autosomal dominant cone dystrophy. Mol Cell 1998; 2: 129–133.
Jacobson SG, Apathy PP, Parel J-M. Rod and cone perimetry: computerized testing and analysis. In: Heckenlively J, Arden GB, eds. Principles and Practice of Clinical Vision Testing. St. Louis, MO: Mosby-Year Book; 1991: 475–482.
Jacobson SG, Cideciyan AV, Huang Y, et al. Retinal degenerations with truncation mutations in the cone-rod homeobox (CRX) gene. Invest Ophthalmol Vis Sci 1998; 39: 2417–2426.
Kremers J, Usui T, Scholl HP, Sharpe LT. Cone signal contributions to electroretinograms in dichromats and trichromats. Invest Ophthalmol Vis Sci 1999; 40: 920–930.
Kretschmann U, Seeliger M, Ruether K, Usui T, Zrenner E. Spatial cone activity distribution in diseases of the posterior pole determined by multifocal electroretinography. Vision Res 1998; 38: 3817–3828.
Kretschmann U, Stilling R, Ruther K, Zrenner E. Familial macular cone dystrophy: diagnostic value of multifocal ERG and two-color threshold perimetry. Graefes Arch Clin Exp Ophthalmol 1999; 237: 429–432.
Sakaue H, Katsumi O, Mehta M, Hirose T. Simultaneous pattern reversal ERG and VER recordings. Invest Ophthalmol Vis Sci 1990; 31: 506–511.
Rabin J, Switkes E, Crognale M, et al. Visual evoked potentials in three-dimensional color space: Correlates of spatiochromatic processing. Vision Res 1994; 34: 2657–2626.
Jacobson DM, Thompson HS, Bartley JA. X-linked progressive cone dystrophy. Clinical characteristics of affected males and female carriers. Ophthalmology 1989; 96: 885–895.
Sandberg MA, Berson EL, Ariel M. Visually evoked response testing with a stimulator-ophthalmoscope. Macular scars, hereditary macular degenerations, and retinitis pigmentosa. Arch Ophthalmol 1977; 95: 1805–1808.
Bodis-Wollner I, Feldman RG, Guillory SL, Mylin L. Delayed visual evoked potentials are independent of pattern orientation in macular disease. Electroencephalogr Clin Neurophysiol 1987; 68: 172–179.
Lennerstrand G. Delayed visual evoked cortical potiential in retinal disease. Acta Ophthalmol 1982; 60: 497–504.
Bass SJ, Sherman J, Bodis-Wollner I, Nath S. Visual evoked potentials in macular disease. Invest Ophthalmol Vis Sci 1985; 26: 1071–1074.
Celesia GG, Kaufman D. Pattern ERGs and visual evoked potentials in maculopathies and optic nerve diseases. Invest Ophthalmol Vis Sci 1985; 26: 726–735.
Kriss A, Russell-Eggitt I. Electrophysiological assessment of visual pathway function in infants. Eye 1992; 6: 145–153.
Lavy T, Harris CM, Shawkat F, Thompson D, Taylor D, Kriss A. Electrophysiological and eye-movement abnormalities in children with the Bardet-Biedl syndrome. J Pediatr Ophthalmol Strabismus 1995; 32: 364–367.
Murray I.J, Parry NRA, Carden D, Kulikowski JJ. Human visual evoked potentials to chromatic and achromatic gratings. Clin Vis Sci 1987; 1: 231–244.
Crognale MA, Kelly JP, Weiss AH, Teller DY. Development of the spatio-chromatic visual evoked potential (VEP): A longitudinal study. Vision Res 1998; 38: 3283–3292.
Porciatti V, Sartucci F. Normative data for onset VEPs to redgreen and blue-yellow chromatic contrast. Clin Neurophysiol. 1999; 110: 772–781.
Crognale MA, Switkes E, Rabin J, et al. Application of the spatiochromatic visual evoked potential to detection of congenital and acquired color-vision deficiencies. J Opt Soc Am A 1993; 10: 1818–1825.
Crognale MA, Rabin J, Switkes E, Adams AJ. Selective loss of S-pathway sensitivity in central serous choroidopathy revealed by spatio-chromatic visual evoked cortical potentials (VECP). In: Drum B, ed. Colour Vision Deficiences XI. Dordrecht: Kluwer Academic Publishers; 1992: 239–249.
Crognale MA, Switkes E, Rabin J, et al. Objective assessment of short wavelength sensitive (SWS) mechanisms with the spatio-chromatic VEP: X-linked achromatopsia and transient tritanopia. In: Drum B, ed. Colour Vision Deficiences XII. Dordrecht: Kluwer Academic Publishers Dordrecht; 1995: 407–413.
Regan D. Evoked potentials specific to spatial patterns of luminance and colour. Vision Res 1973; 13: 2381–2402.
Regan D, Spekreijse H. Evoked potential indications of colour blindness. Vision Res 1974; 14: 89–95.
Berninger TA, Arden GB, Hogg CR, Frumkes T. Separable evoked retinal and cortical potentials from each major visual pathway: preliminary results. Br J Ophthalmol 1989; 73: 502–511.
Marmor MF, Arden GB, Nilsson SEG, Zrenner E. Standard for clinical electroretinography. Arch Ophthalmol 1989; 107: 816–819.
Harding GFA, Odom, JV, Spileers W, Spekreijse H. Standard for visual evoked potentials 1995. Vision Res 1996; 36: 3567–3572.
Beiber ML, Volbrecht VJ, Werner JS. Spectral efficiency measured by heterochromatic flicker photometry is similar in human infants and adults. Vision Res 1995; 35: 1385–1392.
Kelly JP, Chang S. Development of chromatic and luminance detection contours using the sweep VEP. Vision Res 2000; 40:1887–1905.
Cole GR, Hine T. Computation of cone contrasts for color vision research. Behav Res Methods Instrum Comput 1992; 24: 22–27.
Banks M S, Bennett PJ. Optical and photoreceptor immaturities limit the spatial and chromatic vision of human infants. J Opt Soc Am 1988; 5: 2059–2079.
Kelly JP, Borchert K, Teller DY. The development of chromatic and achromatic contrast sensitivity in infancy as tested with the sweep VEP. Vision Res 1997; 37: 2057–2072.
Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol 1991; 78: 378–388.
van der Tweel H, Estevez O. Analytical techniques. In: Heckenlively J, Arden GB, eds. Principles and practice of clinical vision testing. St. Louis, MO: Mosby-Year Book, 1991: 231–253.
Zemon V, Hartmann, EE, Gordon J, Prunte-Glowazki A. An electrophysiological technique for assessment of the development of spatial vision. Optom Vis Sci 1997; 74: 708–716.
Berson EL, Sandberg MA, Rosner B, et al. Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol1985; 99: 240–251.
Berson EL, Gouras P, Hoff M. Temporal aspects of the electroretinogram. Arch Ophthalmol 1969; 81; 207–214.
Berson EL, Sandberg MA, Rosner B, Sullivan PL. Color plates to help identify patients with blue cone monochromatism. Am J Ophthalmol 1983; 95: 741–747.
Reichel E, Bruce AM, Sandberg MA, et al. An electroretinographic and molecular genetic study of X-linked cone degeneration. Am J Ophthalmol 1989; 108: 540–547.
Apkarian P. Visual evoked potential assessment of visual function in pediatric neuroophthalmology. In Albert DM, Jakobeic FA, eds. Principles and practice of ophthalmology, basic sciences. Philadelphia, PA: W.B. Saunders & Co. 1994: 622–647.
Rosenberg ML, Jabbari B. Nystagmus and visual evoked potentials. Neuro-Ophthalmology 1987; 7: 133–138.
Arlt A, Zangemeister WH. Influcence of slow eye movements and nystagmus on pattern induced visual evoked potentials. Neuro-Ophthalmology 1990; 10: 241–251.
Haegerstrom-Portnoy G, Schneck ME, Verdon WA, Hewlett SE. Clinical vision characteristics of the congenital achromatopsias.II. Color vision. Optom Vis Sci 1996; 73: 457–465.
Krill AE, Deutman AF, Fishman M. The cone degenerations. Doc Ophthalmol 1973; 35: 1–80.
Sadowski B, Zrenner E. Cone and rod function in cone degenerations. Vision Res 1997; 37: 2303–2314.
Switkes E, Crognale MA, Rabin J, Schneck ME, Adams AJ. Reply to 'specificity and selectivity of chromatic visual evoked potentials'. Vision Res 1996; 36: 3403–3405.
Klingaman RL. A rod monochromat's pattern VECP at high and low background levels: spatial tuning of colour mechanisms and rods compared. Ophthalmic Physiol Opt 1984; 4: 139–142.
Benedek G, Janaky M, Adamkovich N, Rubicsek G, Sary G. Scotopic pattern-reversal visual evoked potentials. Clin Vis Sci 1993; 8: 47–54.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kelly, J.P., Crognale, M.A. & Weiss, A.H. ERGs, cone-isolating VEPs and analytical techniques in children with cone dysfunction syndromes* . Doc Ophthalmol 106, 289–304 (2003). https://doi.org/10.1023/A:1022909328103
Issue Date:
DOI: https://doi.org/10.1023/A:1022909328103