Abstract
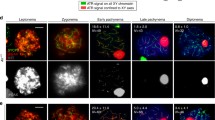
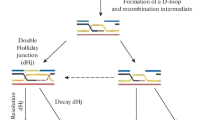
During meiotic prophase 1, homologous recombination is accompanied by dynamic chromosomal changes. The Ce-rdh-1/rad-51 gene is the only bacterial recA-like gene in the nematode C. elegans genome. Upon depletion of Ce-rdh-1/rad-51 using the RNA interference method, abnormal ‘kinked’ chromosomes can be observed in mature oocytes at diakinesis, whereas synapsis between homologous chromosomes during the pachytene stage is normal. Following fertilization, Ce-rdh-1/rad-51-depleted embryos die early in embryogenesis, and their nuclei exhibit abnormal chromosome fragments and bridges. From epistasis analyses with Ce-spo-11 defective mutant and ionizing radiation, it is indicated that Ce-rdh-1/rad-51 functions after double-strand break (DSB) formation of meiotic recombination. Under the Ce-chk-2 defective condition, whose meiotic synapsis and meiotic recombination between homologous chromosomes are completely inhibited, the Ce-rdh-1/rad51 is normally expressed in the gonadal cells. Moreover, it seems that exogenous DSBs in the Ce-chk-2 defective nuclei at the pachytene stage can be repaired between sister chromatids in a Ce-rdh-1/rad-51-dependent manner. These results indicate that Ce-rdh-1/rad51 functions after both endogenous and exogenous DSB formation during meiosis, but not as ‘pairing centers’ for meiotic synapsis.
Similar content being viewed by others
References
Bailis JM, Roeder GS (1998) Synaptonemal complex morphogenesis and sister-chromatid cohesion require Mek1-dependent phosphorylation of a meiotic chromosomal protein. Genes Dev 12: 3551-3563.
Bascom-Slack CA, Ross LO, Dawson DS (1997) Chiasmata, crossovers, and meiotic chromosome segregation. Adv Genet 35: 253-284.
Baudat F, Manova K, Yuen JP, Jasin M, Keeney S (2000) Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6: 989-998.
Bullard SA, Kim S, Galbraith AM, Malone RE (1996) Double strand breaks at the HIS2 recombination hot spot in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 93: 13054-13059.
Cao L, Alani E, Kleckner N (1990) A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell 61: 1089-1101.
Chin GM, Villeneuve AM (2001) C. elegans mre-11 is required for meioticrecombination and DNA repair but is dispensable for the meiotic G(2) DNA damage checkpoint. Genes Dev 15: 522-534.
Cummings WJ, Zolan ME (1998) Functions of DNA repair genes during meiosis. Curr Top Dev Biol 37: 117-140.
de Massy B, Rocco V, Nicolas A (1995) The nucleotide mapping of DNA double-strand breaks at the CYS3 initiation site of meiotic recombination in Saccharomyces cerevisiae. EMBO J 14: 4589-4598.
Dernburg AF, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve AM (1998) Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94: 387-398.
Diaz RL, Alcid AD, Berger JM, Keeney S (2002) Identification of residues in yeast Spo11p critical for meiotic DNA double-strand break formation. Mol Cell Biol 22: 1106-1115.
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-strandedRNAin Caenorhabditis elegans. Nature 391: 806-811.
Gupta RC, Golub E, Bi B, Radding CM (2001) The synaptic activity of HsDmc1, a human recombination protein specific to meiosis. Proc Natl Acad Sci USA 98: 8433-8439.
Higashitani A, Aoki H, Mori A, Sasagawa Y, Takanami T, Takahashi H (2000) Caenorhabditis elegans Chk2-like gene is essential for meiosis but dispensable for DNA repair. FEBS Lett 485: 35-39.
Hong EL, Shinohara A, Bishop DK (2001) Saccharomyces cerevisiae Dmc1 protein promotes renaturation of single-strand DNA (ssDNA) and assimilation of ssDNA into homologous super-coiled duplex DNA. J Biol Chem 276: 41906-41912.
Hubbard EJ, Greenstein D (2000) The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn 218: 2-22.
Keeney S (2001) Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol 52: 1-53.
Keeney S, Giroux CN, Kleckner N (1997) Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375-384.
Kleckner N (1996) Meiosis: how could it work? Proc Natl Acad Sci USA 93: 8167-8174.
Lewis JA, Fleming JT (1995) Basic culture methods. Meth Cell Biol 48: 3-29.
Li Z, Golub EI, Gupta R, Radding CM (1997) Recombination activities of HsDmc1 protein, the meiotic human homolog of RecA protein. Proc Natl Acad Sci USA 94: 11221-11226.
Loidl J, Klein F, Scherthan H (1994) Homologous pairing is reduced but not abolished in asynaptic mutants of yeast. J Cell Biol 125: 1191-1200.
MacQueen AJ, Villeneuve AM (2001) Nuclear reorganization and homologous chromosome pairing during meiotic prophase require C. elegans chk-2. Genes Dev 15: 1674-1687.
Masson JY, West SC (2001) The Rad51 and Dmc1 recombinases: a non-identical twin relationship. Trends Biochem Sci 26: 131-136.
McKim KS, Hayashi-Hagihara A (1998) mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev 12: 2932-2942.
Mitchell A (2001) Meiosis: Synapsis spoilt. Nat Rev Mol Cell Biol 2: 5.
Moens RB (ed.) (1987) In: Meiosis.Orlando, Florida: Academic Press, 1-391.
Molnar M, Bahler J, Kohli J, Hiraoka Y (2001) Live observation of fission yeast meiosis in recombination-deficient mutants: a study on achiasmate chromosome segregation. J Cell Sci 114: 2843-2853.
Nairz K, Klein F (1997) mre11S —a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes Dev 11: 2272-2290.
Oishi I, Iwai K, Kagohashi Y et al.(2001) Critical role of Caenorhabditis elegans homologs of Cds1 (Chk2)-related kinases in meiotic recombination. Mol Cell Biol 21: 1329-1335.
Pasierbek P, Jantsch M, Melcher M, Schleiffer A, Schweizer D, Loidl J (2001) A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev 15: 1349-1360.
Pittman DL, Cobb J, Schimenti KJ et al.(1998) Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell 1: 697-705. 134 Takako Takanami et al.
Rinaldo C, Ederle S, Rocco V, La Volpe A (1998) The Caenorhabditis elegans RAD51 homolog is transcribed into two alternative mRNAs potentially encoding proteins of different sizes. Mol Gen Genet 260: 289-294.
Rinaldo C, Bazzicalupo P, Ederle S, Hilliard M, La Volpe A (2002) Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics 160: 471-479.
Roeder GS (1997) Meiotic chromosomes: it takes two to tango. Genes Dev 11: 2600-2621.
Romanienko PJ, Camerini-Otero RD (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 6: 975-987.
Schedl T (1997) Developmental genetics of the germ line.In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, eds. C. ELEGANS II.Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, pp 241-270.
Shinohara A, Ogawa T (1995) Homologous recombination and the roles of double-strand breaks. Trends Biochem Sci 20: 387-391.
Shinohara A, Ogawa T (1998) Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature 391: 404-407.
Shinohara A, Gasior S, Ogawa T, Kleckner N, Bishop DK (1997) Saccharomyces cerevisiae recA homologues RAD51 and DMC1 have both distinct and overlapping roles in meiotic recombination. Genes Cells 2: 615-629.
Sung P (1994) Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science 265: 1241-1243.
Takanami T, Sato S, Ishihara T, Katsura I, Takahashi H, Higashitani A (1998) Characterization of a Caenorhabditis elegans recA-like gene Ce-rdh-1 involved in meiotic recombination. DNA Res 5: 373-377.
Takanami T, Mori A, Takahashi H, Higashitani A (2000) Hyper-resistance of meiotic cells to radiation due to a strong expression of a single recA-like gene in Caenorhabditis elegans. Nucleic Acids Res 28: 4232-4236.
Tsubouchi H, Ogawa H (2000) Exo1 roles for repair of DNA double-strand breaks and meiotic crossing over in Saccharomyces cerevisiae. Mol Biol Cell 11: 2221-2233.
Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T (1998) The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell 1: 707-718.
Zetka MC, Kawasaki I, Strome S, Muller F (1999) Synapsis and chiasma formation in Caenorhabditis elegans require HIM-3, a meiotic chromosome core component that functions in chromosome segregation. Genes Dev 13: 2258-2270.
Zickler D, Kleckner N (1999) Meiotic chromosomes: integrating structure and function. Annu Rev Genet 33: 603-754.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takanami, T., Mori, A., Takahashi, H. et al. Caenorhabditis elegans Ce-rdh-1/rad-51 functions after double-strand break formation of meiotic recombination. Chromosome Res 11, 125–135 (2003). https://doi.org/10.1023/A:1022863814686
Issue Date:
DOI: https://doi.org/10.1023/A:1022863814686